Laboratory products
New Dissolution Tester has the Edge in Enhanced Mechanical Calibration
Jul 09 2010
Copley Scientific has launched the Dissolution Tester DIS-EMC, a new system that features Enhanced Mechanical Calibration, and which is designed to meet the latest and most rigorous calibration needs in this field.
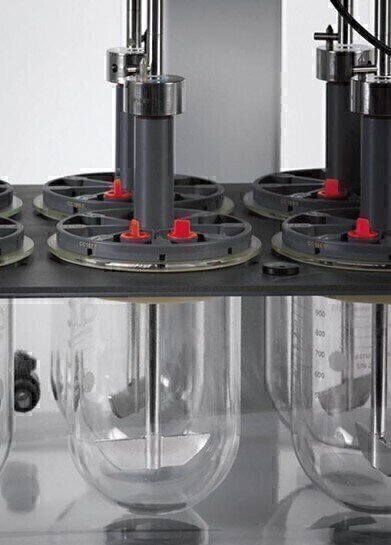
State-of-the-art manufacturing procedures bring new standards in controlling the tolerances and dimensions of the critical elements of the Test Station in which the dosage form resides. In line with current regulatory
guidance, this significantly reduces the potential of the dissolution tester to contribute to testing variability.
While accepted practice in the pharmaceutical industry has been to calibrate dissolution testers using a combination of mechanical checks and performance verification tablets, there is concern about the wide acceptance ranges and variability these generate. As a result the move is towards enhanced mechanical calibration as an alternative, or at least as a precursor, to chemical calibration.
Enhanced mechanical calibration requires strict control of the dimensions of, and the spatial relationships between, critical elements of the dissolution tester. Copley Scientific has identified these critical elements to be the dissolution vessel and lid, and the stirring element. Controlling these, while also maintaining stirring speed and media composition and temperature within tight limits, minimises any instrument contribution to test method variability. Using vacuum forming techniques, Copley Scientific has produced a vessel with superior dimensions and which has provided a platform for the development of a new lid and stirring element. This production method guarantees an internal diameter tolerance and blemish-free spherical radius of +/- 0.13 mm (compared with the traditional unit’s +/- 2 mm). Friction-free bearings in the lid and a precision-ground shaft for the stirring element have enabled a 50% reduction in tolerances relating to wobble, verticality and centring. The Dissolution Tester DIS-EMC betters the dimensional tolerances specified by the FDA by a factor of 2.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh