Laboratory products
New video shows direct server connectivity for Refractometer & Polarimeter - FDA Reg. 21 CFR Pt 11
Sep 18 2019
Computer systems, including analytical instruments with on-board processors that store or produce data to make quality control decisions, or reporting data for the FDA must comply with 21 CFR 11. This includes any laboratory results used to determine quality, safety, strength, efficacy, or purity.
Because FDA reg. 21 CFR part 11 states that closed computer systems must have a collection of technological and procedural controls to protect data within the system, Xylem brand Bellingham + Stanley refined a selection of its instruments to help customers easily comply, including the ADP450 and ADP600 Series of polarimeters as well as the RFM900 Series refractometers.
In short, these products offer the following key benefits for 21 CFR 11.
- Compliance without an intermediate PC reducing data security risk and overall cost of ownership
- Direct FTP link from PC/LIMS/Server to instrument memory for data inspection and transfer
- csv or XML output strings with encryption & MD5 check for easy connection direct to LIMS/Server
- Server synchronised clock to prevent data tampering
- Configurable users enforcing unique login & signature credentials as well as strict password controls
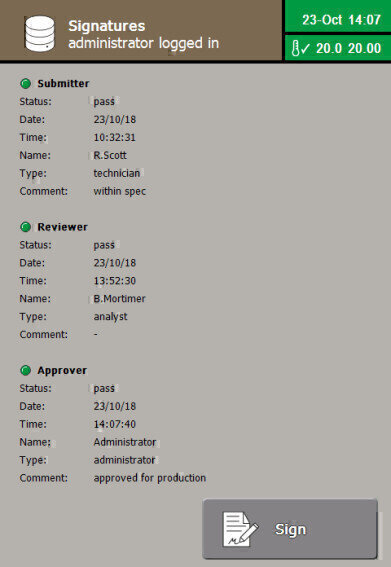
- Local or remote electronic signatures including multi-verification
- Full instrument configuration audit trail
- Print to Secure PDF
- Instrument Configuration Back-up and Clone
Importantly, Bellingham + Stanley’s approach to its common instrument on-board software enhances data integrity whilst eliminating the significant costs involved with PC based software that requires additional validation and control.
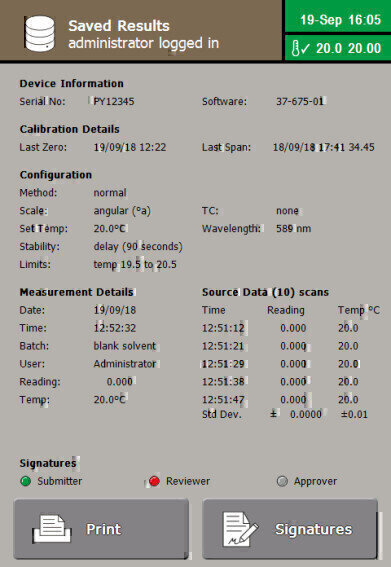
The recording of results, and the logging of instrument access and configuration is also possible. The Review & Approve electronic signature process can even be achieved locally or remotely. Encrypted Print to Secure PDF, .csv and .XML outputs makes data integration to LIMS simple, secure and auditable.
Polarimetry performs an important role in both pharmaceutical and cosmetic manufacturing; from raw materials testing to final quality control.
Bellingham + Stanley’s ADP450 polarimeter incorporating XPC Technology (Xylem’s on-board Peltier system) and the latest software features satisfies all requirements of pharmacopeia (BP/EP/USP) for single wavelength, sodium (589nm) measurement.
For laboratories looking for that extra accuracy or the ability of measuring at other wavelengths, the flagship ADP600 Series polarimeters offer all of the above as well as 3-decimal place accuracy and a variety of pharmacopoeia cited wavelengths across the UV and visual spectrum. Available variants include: 325, 365, 405, 436 and 546nm as well as the newly added 633nm wavelength, equivalent to a helium neon laser line.
The ADP600 Series incorporates an integral airflow Peltier temperature control system that stabilises the sample temperature at the pharmacopeia prescribed 20 or 25°C and now has the latest software for operation in FDA controlled environments. As such, the ADP600 Series meets all of the requirements of pharmacopeia for optical and specific rotation (precision, wavelength, temperature), including the ability to measure Dextromethorphan Hydrobromide at 325nm, where readings must be within 1% of readings of a USP Reference Standard of the same material.
Contact us today for more information or watch the latest video describing how we easily facilitate FDA Regulation 21 CFR Part 11 compliance when using our refractometers and polarimeters.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh