-
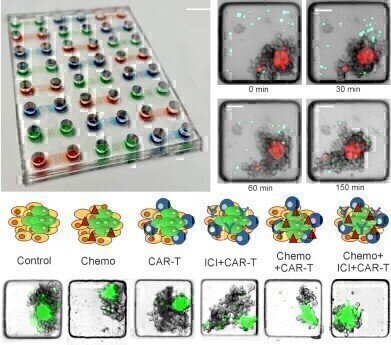 Top left: ONCO-Chip 3D microfluidic plate. Top right: time sequence showing CAR-T cells interacting with 3D tumour models. Bottom: schematic and microscopy images of CAR-T killing cancer cells only (unlabelled) in different combination treatments.
Top left: ONCO-Chip 3D microfluidic plate. Top right: time sequence showing CAR-T cells interacting with 3D tumour models. Bottom: schematic and microscopy images of CAR-T killing cancer cells only (unlabelled) in different combination treatments. -
 Custom CAR-T development by AMSBIO.
Custom CAR-T development by AMSBIO.
Laboratory products
Novel Assay Platform for 3D Microfluidic Cancer Research
Oct 12 2021
Chimeric antigen receptor (CAR-T) cells are genetically modified T-cells that are engineered to find and kill cancer cells by targeting specific cancer-associated proteins, or antigens. CAR-T cell therapy is highly effective against haematological malignancies, but faces challenges in solid tumours due to the immunosuppressive effects of the tumour microenvironment. Often, combination therapies, such chemotherapy and checkpoint blockage, are used with CAR-T to improve efficacy.
The University of Strathclyde (UoS) in Glasgow, and ScreenIn3D Ltd, are using custom CAR-T products supplied by AMSBIO to perform novel immune-oncology assays in 3D microfluidic cancer models.
To investigate CAR-T efficacy, their off-target cytotoxicity and synergistic effects when used in combination assays, UoS and ScreenIn3D researchers developed novel miniaturised screening assays that use very small amounts of CAR-T cells in 3D complex in vitro models of solid tumours on a chip.
This exciting technological advance by UoS and ScreenIn3D researchers is described in detail in a recently published technical article: https://bit.ly/3iASDy0
AMSBIO offers a custom service that enables researchers to take advantage of the astonishing clinical breakthroughs achieved with CAR-T cells in various haematological malignancies. Drawing upon its expertise in monoclonal antibody development (rabbit and mouse), AMSBIO can help you design, plan and execute your CAR-T study, whether you are in the preclinical, clinical or proof of concept stage. The AMSBIO CAR-T platform is highly adaptable to your needs and starting materials, allowing you to start with a target molecule (Phase I) or antibody (Phase II). As part of their custom CAR-T development service, AMSBIO construct the single chain variable fragment (ScFv), transfer it into a CAR lentivector of your choice, make lentivirus and transduce activated human (or mouse) T cells. After the CAR-T cells proliferate, the cytotoxicity is measured in a real time assay, CAR expression analysed and cytokine production quantified.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















