Clinical, medical and diagnostics
New Molecular Diagnostics Website Unveiled
Jan 13 2016
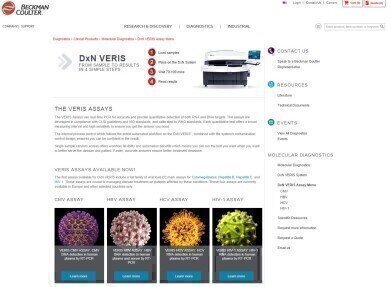
Beckman Coulter Diagnostics has launched its new website specifically for molecular diagnostics. The new site beckmancoulter.com/moleculardiagnostics has been designed not only to showcase the company’s DxN VERIS Molecular Diagnostics System but also to provide quick and easy access to scientific resources and educational materials for the molecular diagnostics laboratory.
“Our aim with the new site has been to make finding information about our molecular diagnostics products, both the DxN VERIS system and its growing portfolio of assays, quick and simple,” said Jaime Menendez-Humara, Director Global Marketing for Molecular Diagnostics, “We are also dedicated to building a dynamic and evolving platform to enable easy access to new scientific and educational materials that will assist our customers and those who wish to learn more about the unique workflow and cost benefits of our molecular diagnostic products.”
“For decades, Beckman Coulter has been at the forefront of laboratory diagnostics, and through significant investment and strategic input from our parent company, the Danaher Corporation, and the expertise of our R&D scientists, we now have a unique offering in molecular diagnostics,” commented Richard Creager, Senior Vice President of Molecular Diagnostics and CSO of Beckman Coulter Diagnostics. “The DxN VERIS consolidates extraction, amplification and detection into one platform, immediately saving operator time, space and costs. When you combine this with the system’s true single-sample, random-access capability that allows multiple assays and samples to be run at the same time, workflow efficiencies lead to greater laboratory productivity, something that is being called for in laboratories worldwide,” he concluded.
The system is fully CE-marked, with assays for Human Immunodeficiency Virus (HIV-1), Hepatitis C Virus (HCV), Hepatitis B Virus (HBV) and Human Cytomegalovirus (CMV). Further menu expansion will follow with many other assays currently in development. Assays are supplied in a unique, single cartridge system so, unlike traditional batch-plate systems, there are no empty wells, reducing wastage and consumable costs. All consumables and reagents are on-board, so there is no lengthy set-up and tests can be up and running in less than 10 minutes with auto-verified results in 70 minutes.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh