-
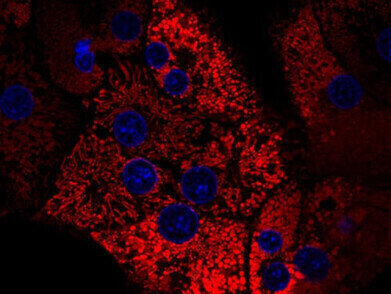 Rat hepatocytes isolated using Collagenase NB 4G (Proved Grade): mitochondria stained with TMRM (red), nuclei stained with Hoechst 33342 (blue). Credit: Dr Pless, University Essen
Rat hepatocytes isolated using Collagenase NB 4G (Proved Grade): mitochondria stained with TMRM (red), nuclei stained with Hoechst 33342 (blue). Credit: Dr Pless, University Essen
Clinical, medical and diagnostics
GMP-grade enzymes streamline clinical cell isolation
Jul 07 2025
Amsbio, in collaboration with Nordmark Biochemicals, has broadened access to high-purity collagenase and neutral protease enzymes, now available in GMP-grade formats designed to meet the stringent demands of clinical tissue dissociation protocols.
These enzymes - sourced from Clostridium histolyticum - play a vital role in the isolation of viable cells from dense or complex tissue samples, a foundational step in regenerative medicine, cell therapy, and tissue engineering. The enhanced GMP-grade versions ensure researchers and clinicians alike can rely on robust, reproducible workflows that comply with evolving regulatory expectations.
Manufactured in facilities adhering to EU GMP standards and additional global quality frameworks - including the FDA’s CFR and ISO guidelines - these enzymes are backed by comprehensive quality control processes. Each lot undergoes rigorous testing to guarantee consistency, with verified virus inactivation and TSE safety certification.
Importantly, the enzymes have been incorporated into recent GMP-compliant protocols for mesenchymal stem cell isolation and production, as documented in studies such as Williams et al. (2025), further validating their suitability for clinical applications.
To support translational workflows, Amsbio offers both research-grade and GMP-grade versions of these enzymes with matching activity profiles. This allows users to develop and optimise methods with research-grade reagents, then seamlessly move to GMP products without revalidating their processes - a significant advantage in accelerating time to clinic.
For scientists advancing cellular therapies from bench to bedside, these clinically validated enzymes offer reliability, flexibility, and regulatory peace of mind.
More information online
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh