Chromatography
Simplifying Methods Transfer: Novel Tools for Replicating your Established Methods on the ACQUITY Arc System
Sep 17 2015
Retention shifts in methods transfer are commonly observed. Retention can be impacted by differences in system and pump characteristics such as dwell volume and mixing characteristics. Many laboratories require there be no adjustments to a method, particularly for validated methods. If adjustments are necessary, consideration is generally made to be in accordance with regulatory guidelines. With reference to dwell volume, chapter <621> of the USP monograph states "If adjustments are necessary, change in ... the duration of an initial isocratic hold (when prescribed), and/or dwell volume adjustments are allowed [1]". While adjustments for dwell volume can be manually entered into a gradient table, this approach requires calculations and manual changes to the gradient table; both of which require additional time and effort. Using a feature in the software to adjust the time between the start of the gradient and the point of injection, the duration of the gradient hold can be adjusted. This is accomplished without the need to make changes to the gradient table allowing systems - such as the ACQUITY ArcTM System- to mimic systems with different dwell volumes. By being able to enter the value directly in time, methods transfer can be streamlined by measuring the difference in retention time and adjusting the initial hold accordingly.
[for the full application note, please visit www.waters.com and search for 7200005469en, or here is the direct link http://www.waters.com/waters/library.htm?cid=511436&lid=134856832}
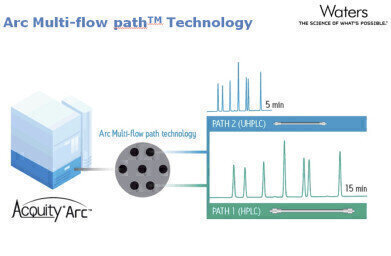
The ACQUITY Arc system minimises downtime and inefficiencies, allowing the seamless transfer of new technology into regulated environments. Enabled by the Arc multi-flow path™ technology, the ACQUITY Arc System can easily replicate methods developed on previous generations of LC instrumentation without alteration to the method. By simply selecting Path 1 or Path 2 in the system software, the user can emulate the dwell volume and mixing behaviour of their legacy HPLC systems in efforts to replicate their established HPLC methods without the need for revalidation.
The Arc multi-flow path technology was designed specifically to enable users to successfully transfer their established HPLC methods on to a modern LC platform, while providing them the flexibility to further improve their productivity when pairing the system with a more modern UHPLC column technology.
[For information on the ACQUITY Arc System, please visit www.waters.com/arc]
REFERENCES:
1. In United States Pharmacopeia and National Formulary (USP 37-NF 32 S2). ; United Book Press, Inc.: Baltimore, MD, 2014; Vol. , p 6376.
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh