-

-
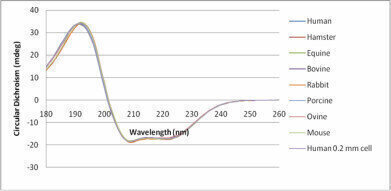 Figure 1. Circular Dichroism spectra of various mammalian serum albumins normalised by area to that of human serum albumin (HSA). Note the small shift in the ~190 nm peak.
Figure 1. Circular Dichroism spectra of various mammalian serum albumins normalised by area to that of human serum albumin (HSA). Note the small shift in the ~190 nm peak.
Chromatography
Biocomparability studies now in the domain of Circular Dichroism spectroscopy
Sep 07 2011
A biotherapeutic must maintain stability throughout storage until delivery to a patient. Preparing samples and experiments to test the properties is highly labour intensive, requires multiple analytical techniques and is prone to sample error. Besides, many proteins have a propensity to slowly aggregate and precipitate with time during storage thereby representing a significant loss of profit.
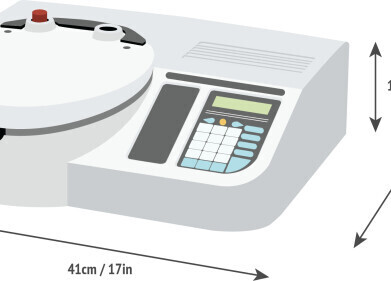
The automated “Chirascan™-plus ACD” CD spectrometer enables rapid, accurate, and highly reproducible measurements for the study of functionally-related aspects of protein structure which has proven to be an exceptionally good indicator of the relative stability of biotherapeutics in solution. Biocomparability studies using Chirascan™-plus ACD can quickly identify the stability of a drug product when compared to a standard, thus allowing delivery of a drug product in its native state.
The Chirascan™-plus ACD has been developed to enable unattended high throughput running of 96 and 384 microplates with washing, drying and measuring cycles that guarantees elimination of sample dilution and cross contamination. Typical studies include the influence of pH, ionic strength, detergent, mutation and ligand binding on protein conformation, important in biotherapeutic development, formulation testing and clone selection procedures.
This innovative technology simplifies the complexity around process development for biosimilar products by:
• offering advanced sample & experiment design
• sequentially delivering samples to the spectrometer from a microtitre plate with effectively zero inter-sample carry-over
• automatically acquiring and storing spectroscopic data for up to 384 samples without operator intervention
• recovering samples to minimize cost and maximize production
• simultaneously measuring more than one optical property (e.g. CD, fluorescence and absorption) to maximize the information return per sample
• interfacing seamlessly with the data analysis software designed to simplify the interpretation of spectroscopic data
The robustness of the technique and data quality is demonstrated in a recent study, Automated Comparison of Circular Dichroism Spectra of Proteins.
Figure 1. Circular Dichroism spectra of various mammalian serum albumins normalised by area to that of human serum albumin (HSA). Note the small shift in the ~190 nm peak.
The automated sample preparation and measurement of CD now enables experiments to be undertaken that were previously thought to be too time consuming or laborious, with applications such as pH and ionic strength titrations, protein stability experiments and biocomparability studies now very much in the domain of Circular Dichroism.
Click here to contact us to find out more:
E: sales@photophysics.com
W: www.photophysics.com
T: 0044 (0) 1372 386537
USA: 1-800 543 4130
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh