-
 Quality control during production of LCK Cuvette Tests.
Quality control during production of LCK Cuvette Tests. -
 Addista Multi-Parameter Standard Solutions for internal and external control.
Addista Multi-Parameter Standard Solutions for internal and external control. -
Laboratory Products
How do you make sure your analytical results are always correct?
Nov 02 2016
The quality of goods and services is crucially important. Purchasers and users have come to expect high quality standards from suppliers and manufacturers. This is why quality is checked and documented several times over. Results of analyses also have to be able to prove their quality. However, there is much more to ensuring high quality measurement results than simply the type of method (standard/norm vs. operational analysis) that is used. The care taken over the individual worksteps and the quality assurance measures that are implemented, play a much greater role:
Product Quality + Workflow Quality + AQA Measures = Quality Results
Lab workers and their superiors are liable for any incorrect interpretations and decisions that are made as a consequence of incorrect analysis results. Integrating appropriate quality control measures at the relevant points of the analysis process ensures reliable results and minimises the risk of exposure to liability.
How quality assurance is organised in the laboratory
Organising and carrying out AQA in laboratories involves dealing with a variety of international and local standards. Central points are:
- Defining internal and external measures to be implemented based on applicable standards
- Monitoring and maintaining analytical equipment
- Training laboratory staff
- Documenting implemented measures and results
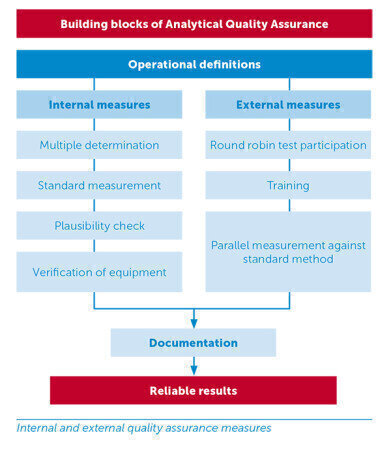
Building blocks of quality control
- Internal quality assurance – This is carried out by the user themselves. Measures include multiple determination, standard measurement, plausibility check, veri?cation of equipment
- External quality assurance – This results from collaboration between user and manufacturer or between different laboratories. Measures include round robin test participation, parallel measurement, training
- Documentation – AQA starts with taking a sample and ends with an analysis report in the laboratory logbook. Documentation must be accurate and clearly arranged. It must be obvious who produced what analysis data and when. All results of quality assurance measures should be entered onto the relevant control cards, see picture.
The operational de?nitions (de?nition of measures, frequency and quality control objectives) ensure that individual measures are tailored to suit the needs of the plant.
What do you need to pay attention to?
- All measured results should be within the con?dence interval.
- Aim to improve working methods by narrowing the con?dence interval.
- Observe trends.
- Analysis values and empirical values must also match.
Conclusion
Regular application of AQA ensures that:
- The results of analyses are traceable.
- The correct status of the analysis system is documented.
- Handling errors can be recognised immediately.
- Comparison of measured results is possible.
- Results of analyses are recognised.
Giving you confidence for the important decisions
Hach® supports users of LCK Cuvette Tests by carrying out a substantial part of QA measures on their behalf. Relevant quality and batch certificates are available, e.g. on the Hach websites. Addista Multi-Parameter Standard Solutions provide internal and external QC. Application experts support users when carrying out individual quality control measures.
Download the AQA Guide with a glossary of measures and recommendations for frequency and quality targets.
Digital Edition
International Labmate Buyers' Guide 2024/25
June 2024
Buyers' Guide featuring: Product Listings & Manufacturers Directory Chromatography Articles - Enhancing HPLC Field Service with fast-response, non-invasive flowmeters - Digital transformatio...
View all digital editions
Events
Jul 03 2024 Gandhinagar, India
Jul 07 2024 Dublin, Ireland
Jul 20 2024 Denver, CO, USA
Jul 21 2024 Cape Town, South Africa
Jul 28 2024 San Diego, CA USA