Analytical Instrumentation
Distillers corn oil (DCO) : A study of recent advancements in the processing techniques for this unique fuel source
May 07 2024
Author: Dr. Raj Shah, Brajendra K. Sharma, Dr. Kirtika Kohli, Mr. Gavin Thomas, Mr. Nikhil Pai, and Ms. Jacqueline Nowicki on behalf of Koehler Instrument Company
Free to read
Articles are free to download. Unlock the article to be shown more content, graphs and images.
Distillers corn oil (DCO) is a rather unique fuel source. In a world of fossil fuels and electrical power, synthesizing viable mechanical fuel from a food source seems remarkable, if not impossible. While refined corn oil is used mainly as a cooking oil due to its high smoke point, modern technologies have developed processes that transform corn crops into ethanol, an alternative fuel manufactured primarily in the United States[1]. This form of ethanol remains the most produced, with roughly 40% of U.S. corn crops dedicated specifically to ethanol manufacturing[2].
While corn proves to be a stable source of ethanol, DCO is a coproduct of ethanol production. Unlike refined corn oil, historically DCO has not proven to be a viable feedstock for biodiesel due to its relatively high price[3]. However, the U.S. and other countries have begun favoring the use of DCO for biodiesel synthesis, as DCO became the fastest-expanding feedstock for biodiesel in 2013[4]. Since then, corn oil production has experienced remarkable growth and development, as new techniques and methods increase DCO production and further refine corn oil efficiency[2]. The past five years are emblematic of its growth as a fuel source as innovations and production lines have further optimized corn oil’s viability as both a fuel source and a consumable product. This article will detail several advancements made within the last five years, remarking on their impact and positive implications on the future of DCO in both feed and biodiesel production.
DCO Production Methods:
Before noting the developments made towards DCO production, a further understanding of the unique facets of DCO production is pertinent. The first step in any corn oil process is the separation of the corn germ, the portion of a corn kernel containing the oil[3]. There are two primary methods to process corn: wet and dry milling[5-7] (processes are shown in Figure 1).
Wet milling softens the kernels by soaking them and then separating them into their constituent parts through a series of mills, which allows for the extraction of the individual components. The major coproducts of wet mill ethanol process are corn gluten meal (high protein, 40%) and corn gluten feed (low protein, 28%), and corn germ, which can be further processed into corn oil[7]. In contrast, dry milling directly grinds the kernels into flour. The starch in the flour is converted to ethanol during the fermentation process, creating carbon dioxide and distiller grains. DCO is recovered post-fermentation during ethanol production. Rather than extracting oil from the germ prior to fermentation, recovery steps are performed to take advantages of potential economic benefits of oil recovery from the whole or thin stillage. Whole stillage is the remaining liquids with 8-10% solids after ethanol recovery. Thin stillage is the liquid remaining after centrifugation to separate the solids (wet cake). Without oil recovery, thin stillage is concentrated with evaporators to about 30% solids to create a syrup[8]. DCO is separated from this syrup using separators such as tricanters and disc stack separators[9]. In U.S., 57% of corn ethanol facilities used disc stack separator with a DCO recovery system. The solids from the syrup can be mixed with the wet cake and dried to produce dried distiller grains. The co-products i.e., wet distiller grains and dried distiller grains are used as animal feed (Figure 1). Approx. 90% of ethanol plants utilize a dry grind process because of its lower capital costs. In a nutshell, crude corn oil is primarily produced by wet mill ethanol plants whereas DCO is from dry mill ethanol plants.
As seen opposite, both production methods are entwined within existing industries: ethanol production in dry milling, and food processing in wet milling. As a result, both industries inevitably affect and intersect with corn oil production. Countries that already produce and process high amounts of corn domestically also tend to produce and export high amounts of corn oil, due to the presence of existing processing plants and the general ease of implementing further measures of DCO extraction into those existing production lines. As previously mentioned, 40% of the U.S. corn crop is dedicated to ethanol. Unsurprisingly, the U.S. is also the world’s largest producer of DCO, owing to the country’s capacity for corn and its existing systems dedicated to processing and refining crop yield[10].
Furthermore, as DCO is obtained as a coproduct of industrial corn processing rather than a direct product, its growth potential is restrained by the growth of those processes[4]. The growth of ethanol production and wet separation will typically intersect with the growth of DCO processing, and vice-versa. Finally, the viability of corn oil as both a potential biodiesel feedstock and as a cooking ingredient poses several avenues for development. For biodiesel, development is driven towards fuel production, typically to optimize yield or efficiency or to remove imperfections within the product. However, development in the nutritional field focuses on corn oil refinement for consumption, focusing typically on removing contaminants or increasing the yield of corn oil from other sources. This article will focus on several advancements in both fields.
Fatty Acid Ethyl Esters From DCO:
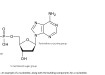
Typically, biodiesel from vegetable oil requires that oil to be refined before the transesterification process. However, refined vegetable oil is more expensive, and contributes heavily towards the cost of the product[7]. Thus, many have attempted to locate cheaper raw materials that fulfill the requirement at a lower price. Regarding biodiesel production pathways, a team of researchers from Brazil synthesized biodiesel directly from the DCO output of corn ethanol production[11]. The distiller grains produced from corn ethanol processes contain a high protein content, which justifies its primary use as an animal feed. Additionally, it contains a high oil content, which can be extracted without reducing the protein content and value of the grain[7]. Furthermore, that oil has a fatty acid profile similar to refined corn oil (RCO), allowing it to function as a raw material[11]. Thus, through bio-catalyzed processes it becomes theoretically possible to enzymatically convert DCO into fatty acid ethyl esters (FAEE) in a single step, thus producing biodiesel without the need to refine the raw material beforehand. In this study, Aguieiras, et al. reported converting DCO into an FAEE through reactions catalyzed by commercial lipases, graphically demonstrated in Figure 2. Five lipases were analyzed and the reactions with the two best-performing biocatalysts were optimized. Additionally, three additional methods to improve the FAEE output were evaluated: ethanol stepwise addition, consecutive reactions, and enzyme mixture. The results of the DCO process were then compared with that of biodiesel produced by RCO, with six batches of each provided to gauge stability.
From the lipase groups used, the team selected Lipozyme TL IM and Novozym 40086 for further optimization. This was selected due to increased enzyme concentration and ethanol:oil molar ratio and their ester content from both biocatalysts being roughly 60%, close to the maximum theoretical yield percent of 66%[11]. Further examination proved that combining both catalysts further improved the yield and reaction time to optimal levels, as shown in Figure 3. Finally, comparing the DCO biodiesel to the RCO biodiesel indicated that DCO produced higher yield of esters as shown in Figure 4. As the process yielded high amounts of ester with a low reaction time, the study confirms DCO as a viable feedstock to generate FAEE for biodiesel production.
The results are significant, as DCO now presents a low-cost alternative to corn oil that remains viable for several reasons. Firstly, the DCO presented in the study is not an edible oil, freeing it from the food vs. fuel controversy corn oil is mired in. RCO is also more expensive and more wasteful than DCO, which is a byproduct of animal feed that is already being produced[11]. Finally, the potential of biodiesel generation from DCO also indicates the possibility of integrating biodiesel production into existing ethanol production plants, which would prove monumental for biodiesel production and corn oil recyclability.
Glycerol Refinement:
While DCO is viable as a biodiesel feedstock, it is limited by a relatively high FFA content compared to other vegetable oils, reducing catalyst activity and restricting the rate of production[11]. Although a high FFA content is a severe issue, the widespread availability of DCO continues to prove incredibly valuable if utilized. Many have sought methods to convert the FFA into fatty acid methyl esters (FAME), including the previously mentioned lipase-catalyzed processes.
While many intriguing pathways exist, most require catalysts, which come with specific issues relating to catalyst compatibility. Thus, the pursuit of catalyst-free procedures has also intrigued many researchers[12]. In this endeavor, another research group from Brazil reported a method to remove the catalyst from the process while retaining DCO as a feedstock[12]. The esterification reaction of FFA with alcohol is a unique method to reduce FFA content. Furthermore, glycerol is a byproduct of biodiesel production[13], meaning that increased biodiesel production has also produced a surplus of glycerol. Thus, the government-backed research group helmed by lead scientist Emilia Monserrat Ortiz Villarruel proposes an esterification method for the FFA present in DCO using glycerol, hopefully establishing a sustainable supply chain due to increased production of both.
The experiment utilizes DCO and distiller glycerol intending to induce esterification to convert the FFA into triglycerides (TAG), noting the effect of reaction conditions in a catalyst-free environment. Following the reaction, the effects of vacuum pressure, glycerol oil/mass ratio, and temperature were all examined concerning the DCO samples.
The stronger vacuum pressure conditions facilitated water removal from the system and prevented reverse equilibrium from contaminating the sample. The glycerol oil/mass ratio directly affected yield, with the study finding a 2:1 ratio to yield optimal TAG levels and FFA reduction. Furthermore, increased temperatures promoted mass transfer, glycerol solubility, and further interactions. Through numerical optimization, FFA content decreased from 18 wt% to 2.12 wt%, and TAG content increased to 43.27 wt%. The accuracy of the model was affirmed, and the study concludes by establishing a sustainable and cost-effective glycerol utilization with DCO FFA as its feedstock[12].
This experiment presents a new catalyst-free method to produce a sustainable feedstock from DCO. If this process can be further optimized and implemented on a widespread scale, DCO can become a reliable and effective feedstock for biodiesel, which promises to bolster biodiesel production. Furthermore, the results can be further explored to produce cost-effective and eco-friendly biodiesel production methods from untapped feedstocks.
Enzymatic Detoxification:
In the field of corn oil for consumption, a new method has been developed to combat the contamination of a uniquely harmful mycotoxin distributed widely in maize and its byproducts[14]. Mycotoxin zearalenone (ZEN) is a widespread contaminant in the industry, as its estrogenic behavior disturbs homeostasis and induces several diseases in both humans and animals who consume it.
ZEN is especially common in maize from countries with warm and wet climates[15], and contamination can occur in planting, harvesting, storage, transportation, and processing given ideal conditions[14]. In particular, corn oil is susceptible to fungal infection and contamination during its industrial milling process, as maize gum presents ideal conditions for ZEN to flourish. While numerous physical strategies to prevent this infection may work, most, if not all methods result in significant loss of nutrients through detoxification, as the indiscriminate removal also results in a loss of vitamin E and sterols. Thus, the need to remove ZEN without harming nutritional value arises.
As a solution, biotechnological methods have been met with enthusiasm, as microbial enzymes naturally eliminate and transform contaminants and can be widely applied in food processing[14]. Several enzymes have already demonstrated their ability to metabolize the ester bonds within ZEN and reduce the compound’s toxicity, as shown in Figure 3, and using similar enzymes in corn oil could prove successful at reducing the toxicity of the product without reducing its nutritional value, as enzymatic activity can break the structure of ZEN without also removing further sterols or leaving toxic byproducts[14].
Thus, a 2020 study by a group of researchers from the Institute of Grain & Oil Science Technology in China released findings detailing the enzymatic removal of ZEN from corn oil during the refining process using the ZEN degrading enzyme ZLHY-6, an enzyme specifically created to degrade ZEN, with attention paid to the loss of total tocopherol and sterol content within the oil during the reaction[14]. In the experiment, the effects of the amounts of degrading enzyme, reaction time, reaction temperature, and mixing strength on ZEN degradation performance were all recorded. Following the reaction and chromatography analysis of the various samples, the research group measured that the tocopherol and sterol content within the sample was reduced by 12.09% and 24.03%, respectively, in only the crude oil sample, meaning that the final product would experience a minimal decrease in both.
The results proved the use of enzymes successful as ZEN concentration reduced from an initial concentration of 1257.3 µg/kg to 13 µg/kg, with the rates of ZEN degradation displayed in Figure 6. Compared to neutralized oil, tocopherol and sterol content did not significantly change, indicating that the process successfully removed ZEN from the corn oil without compromising the nutritious sterols and tocopherols in the original sample. The results of this experiment are significant, as the removal of ZEN from the corn oil through enzymes allows for further refinement and removal of contaminants during corn oil processing. If this method were implemented in processing plants, commercial corn oil would be far safer to consume, with one more contaminant safely and efficiently dealt with.
Neutralization of Acid Corn Oil:
Finally, a 2019 study published by a research group from the Universidad Nacional de Río Cuarto in Argentina promises to reduce the acidity of acid corn oil (ACO), allowing for its reuse as a viable corn oil[16]. A byproduct of corn ethanol production, ACO is a concentrated compound obtained from the centrifuge process that isolates DCO. Like DCO, ACO contains a high FFA content, rendering it inedible and relegating it to be used for biodiesels. Deacidification is possible through various means, such as molecular distillation (MD), which is uniquely capable of the task, as solvents can act as separating agents and do not risk creating highly toxic products[17]. Thus, the study aimed to reduce the acidity of ACO through MD to suitable values for use as edible oil and to analyze its composition of tocopherols, noting the FFA and PI content of the ACO and its product.
The percentage of FFA in the oil was 9.44%, with acid profiles and chromatograms published within the study. From the experiment, the group discovered a positive relation between a higher evaporation temperature and a higher concentration of tocopherol content. Furthermore, as Figure 7 indicates, increasing the distillation temperature, Tw, reduces the fatty acid content in the oil. Ultimately, the study was proven successful, as MD use under a distillation temperature of 190 °C and a volumetric flow, Fv, of 0.5 mL min−1 produces an oil with an acidity lower than 0.3%, falling within the threshold for human consumption. If such a method were industrially applied, the supply chain for corn oil would be bolstered further. With increased corn oil reserved for consumption, more corn oil will be available for biodiesel production.
Conclusions:
As a uniquely multifaceted resource, distillers corn oil development is fractured, with developments relating to many different purposes. As a fuel source, DCO development is defined by updated pathways that produce cleaner feedstocks with cheaper raw materials. As an edible product, corn oil can be refined to eliminate contaminants without compromising nutritional value. Regardless, DCO has improved in many sprawling ways. Newer methods have managed to transform raw DCO into a workable biodiesel feedstock, enzyme treatments have conclusively eliminated a primary contaminant for corn oil as a whole, DCO feedstock conversions can potentially reduce in price by eliminating costly catalysts, and previously unsalvageable acidic corn oil has been recovered into a usable state. These innovations are only a few of the many different methodologies that continue to drive the industry forward, and corn oil as a feedstock and an edible product will continue to refine and flourish until corn oil eventually becomes a viable and sustainable fuel source.
Resources:
1. U.S. Department of Energy. “Ethanol production and distribution.” [Online]. Available: https://afdc.energy.gov/fuels/ethanol_production.html
2. U.S. Department of Agriculture. “Global demand for fuel ethanol through 2030.” [Online]. Available: https://www.ers.usda.gov/publications/pub-details/?pubid=105761
3. Veljković VB, Biberdžić MO, Banković-Ilić IB, et al. “Biodiesel production from corn oil: A review. Renewable & Sustainable Energy Reviews, 2018, 91, 531–48, https://doi.org/10.1016/j.rser.2018.04.024. [Online]. Available: https://www.sciencedirect.com/science/article/abs/pii/S136403211830234X
4. Farm Progress. “Biodiesel Industry Turns to Corn Oil.” 2014. [Online]. Available: https://www.farmprogress.com/technology/biodiesel-industry-turns-to-corn-oil
5. How is ethanol made? Renewable Fuels Association (RFA) https://ethanolrfa.org/ethanol-101/how-is-ethanol-made
6. Vohra M, Manwar J, Manmode R, Padgilwar S, Patil S. Bioethanol production: feedstock and current technologies. Journal of Environmental Chem. Eng. 2014, 2, 573-584, https://doi.org/10.1016/j.jece.2013.10.013
7. Mosier NS, Ileleji KE. Chapter 26- How fuel ethanol is made from corn. Bioenergy (Second Edition) Biomass to Biofuels and Waste to Energy, 2020, 539-544, https://doi.org/10.1016/B978-0-12-815497-7.00026-9
8. Moreau RA, Hums ME. Corn oil and distillers corn oil. Bailey’s Industrial Oil and Fat Products, Seventh Edition, edited by Fereidoon Shahidi. John Wiley & Sons Ltd. 2020, https://doi.org/10.1002/047167849X.bio007.pub2
9. JUV, JBSJ, JWM, LRD, YYZH, MVZ, PKLM. Distiller’s corn oil recovery in ethanol production facilities, https://biosolutions.novozymes.com/sites/default/files/inline-files/Distillers%20Corn%20Oil%20Recovery%20White%20Paper.pdf
10. The Science Agriculture. “10 World’s Biggest Corn Oil Producers.” [Online]. Available: https://scienceagri.com/10-worlds-biggest-corn-oil-producers/
11. Aguieiras, Erika C. G., Abreu, Kévin Enrick A. de, Oliveira, Renata A., et al. Fatty acid ethyl esters production from distillers corn oil by enzymatic catalysis. Journal of the American Oil Chemists’ Society, 2022, 99(5), 393–405, https://doi.org/10.1002/aocs.12582. [Online] Available: https://aocs.onlinelibrary.wiley.com/doi/abs/10.1002/aocs.12582
12. Villarruel EMO, de Carvalho Filho JFS, Garcia MAS, Monnerat J, Aranda DAG, Romano PN. Maintaining high‐triglyceride content and reducing free fatty acids in distillers corn oil: A catalyst‐free multivariate strategy. Journal of the American Oil Chemists’ Society, 2023, https://doi.org/10.1002/aocs.12802. [Online]. Available: https://aocs-onlinelibrary-wiley-com.proxy.library.stonybrook.edu/doi/full/10.1002/aocs.12802
13. Farm Energy. “New uses for crude glycerin from biodiesel production.” 2019. [Online]. Available: https://farm-energy.extension.org/new-uses-for-crude-glycerin-from-biodiesel-production/
14. Chang X, Liu H, Sun J. Zearalenone removal from corn oil by an enzymatic strategy. Toxins, 2020, 12(2), 117-, https://doi.org/10.3390/toxins12020117. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7076758/
15. Kovalsky P, Maria P, Schweiger, W, et al. “Zearalenone-16‑O‑Glucoside: A new masked mycotoxin. Journal of Agricultural and Food Chemistry, 2014, 62(5), 1181–89, https://doi.org/10.1021/jf405627d. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/24386883/
16. Miró Erdmann SM, Magallanes LM, Tarditto LV, et al. “Recovery of acid corn oil from the bioalcohol industry by molecular distillation. Journal of the Science of Food and Agriculture, 2019, 99(9), 4468–73, https://doi.org/10.1002/jsfa.9684. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/30868588/
17. Pina CG, Meirelles AJA. Deacidification of corn oil by solvent extraction in a perforated rotating disc column. Journal of the American Oil Chemists’ Society, 2000, 77(5), 553–59, https://doi.org/10.1007/s11746-000-0088-3. [Online]. Available: https://aocs-onlinelibrary-wiley-com.proxy.library.stonybrook.edu/doi/10.1007/s11746-000-0088-3
About the Authors
Dr. Raj Shah is a Director at Koehler Instrument Company in New York, where he has worked for the last 28 years. He is an elected Fellow by his peers at IChemE, CMI, STLE, AIC, NLGI, INSTMC, Institute of Physics, The Energy Institute and The Royal Society of Chemistry. An ASTM Eagle award recipient, Dr. Shah recently coedited the bestseller, “Fuels and Lubricants handbook”, details of which are available at ASTM’s Long-Awaited Fuels and Lubricants Handbook 2nd Edition Now Available (https://bit.ly/3u2e6GY).He earned his doctorate in Chemical Engineering from The Pennsylvania State University and is a Fellow from The Chartered Management Institute, London. Dr. Shah is also a Chartered Scientist with the Science Council, a Chartered Petroleum Engineer with the Energy Institute and a Chartered Engineer with the Engineering council, UK. Dr. Shah was recently granted the honourific of “Eminent engineer” with Tau beta Pi, the largest engineering society in the USA. He is on the Advisory board of directors at Farmingdale university (Mechanical Technology) , Auburn Univ ( Tribology ), SUNY, Farmingdale, (Engineering Management) and State university of NY, Stony Brook ( Chemical engineering/ Material Science and engineering). An Adjunct Professor at the State University of New York, Stony Brook, in the Department of Material Science and Chemical engineering, Raj also has over 600 publications and has been active in the energy industry for over 3 decades. More information on Raj can be found at https://bit.ly/3QvfaLX
Contact: rshah@koehlerinstrument.com
Brajendra K. Sharma, Research Chemist at ERRC, USDA/ARS/NEA, a fellow of the Royal Society of Chemistry and the Society of Tribology & Lubrication Engineers works in biolubricants, biofuels, alternative fuels, biobased asphalt binders, additives, and chemicals.
Mr. Gavin Thomas and Mr. Nikhil Pai are part of a thriving internship program at Koehler Instrument company in Holtsville, and are students of Engineering at Stony Brook University, Long Island, NY, and University of Texas at Austin respectively.
Dr. Kirtika Kohli, Scientist at CSIR-Indian Institute of Petroleum, Dehradun. She works in catalytic and thermal conversion processes, separation processes, petrochemicals, alternate fuels, and crude to chemicals.
Ms. Jacqueline Nowicki,
QAQC Laboratory Manager at Valero Renewable Fuels in Minnesota. She has 20 years within the quality space, currently specializing in the ethanol field and bi-products. An active member of ASTM D02 and E48 committees.
Free to read
Articles are free to download. Please login to read this article or create an account.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
May 21 2024 Lagos, Nigeria
May 22 2024 Basel, Switzerland
Scientific Laboratory Show & Conference 2024
May 22 2024 Nottingham, UK
May 23 2024 Beijing, China
May 28 2024 Tel Aviv, Israel







.jpg)




