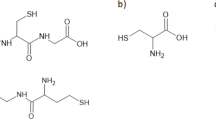
Abstract
The report presents first high performance liquid chromatography coupled with ultraviolet detection (HPLC-UV) based method for the determination of homocysteine thiolactone (HTL) in human urine. Sample preparation procedure chiefly involves chloroform liquid-liquid extraction of HTL and its re-extraction with hydrochloric acid, followed by evaporation to dryness and treatment of the residue with 1-benzyl-2-chloropyridinium bromide and perchloric acid. The chromatographic separation of obtained UV-absorbing 2-S-pyridinium derivative is achieved within 6 min at 25 °C on Zorbax SB-C18 (150 × 4.6 mm, 5 μm) column using gradient elution. The eluent consists of 0.1% acetic acid in a mixture with acetonitrile delivered at a flow rate of 1 mL/min. The analyte is quantified by monitoring at 316 nm. The assay linearity was observed within 0.1-1.0 µmol/L in urine. The limit of quantification was found to be 100 nmol/L. The accuracy ranged from 92.56 to 112.51% and 92.56–114.31%, while precision varied from 5.89 to 11.27% and 3.85–14.25% for intra- and inter-assay measurements, respectively. The method was successfully applied to urine samples delivered by apparently healthy donors. The presence of HTL was confirmed in twelve of eighteen study samples. The HTL concentration ranged from 36.67 to 693.12 nmol/L.
Similar content being viewed by others
Introduction
Homocysteine thiolactone (HTL) is an intramolecular thioester of homocysteine (Hcy) which is recognized to be a cytotoxic metabolite of protein-related Hcy metabolism. Lately, it has been suggested that HTL can be mechanistically involved in cardiovascular diseases due to its chemical reactivity and the ability to modify protein lysine residues1,2,3,4. In a recent prospective studies with a large cohort of coronary artery disease individuals5and stroke patients6, it has been found that elevated urinary HTL is a risk predictor of incident acute myocardial infarction as well as increases the risk of ischemic stroke by promoting unfavorable fibrin clot properties. These findings highlight the necessity for development of a new assays to make further studies of HTL easier in health and disease as its role in humans is not sufficiently examined.
So far, a number of methods have been developed enabling determination of HTL in human urine, plasma and saliva7,8, while analysis of urine is currently of the greatest diagnostic importance1,7. Among several separation techniques, high performance liquid chromatography (HPLC)8,9,10,11, capillary electrophoresis (CE)12,13,14and gas chromatography (GC)15,16coupled with ultraviolet (UV)12,13,14, fluorescence (FLD)9,10,11and (tandem) mass spectrometry detection (MS)/MS8,15,16were used to quantitate HTL in urine. In particular, the methods rely on urine sample purification by liquid-liquid extraction (LLE)8,10,12,15,16, single drop microextraction13,14and solid phase extraction9,11. In addition, some of the assays involve the determination of HTL after on-column or post-column derivatization with o-phthaldialdehyde (OPA)9,10,11, while the other approaches employ pre-column derivatization of HTL with N-trimethylsilyl-N-methyl trifluoroacetamide15and isobutyl chloroformate16. In the case of CE-UV12,13,14and HPLC-MS/MS8 assays, derivatization was not needed to produce signal of the analyte.
Up to date, methods allowing the determination of urinary HTL by HPLC-UV are not available, despite the fact that it is the most commonly used analytical technique in the bioanalysis. Thus, our aim was to provide method for the determination of HTL in human urine by HPLC-UV. Such approach will make possible to widespread use of delivered assay in worldwide laboratories and it should facilitate further studies exploring the role of HTL.
Materials and methods
Reagents and materials
In this study, commercially available chemicals and at least of analytical reagent grade were used. Trisodium phosphate, sodium phosphate dibasic heptahydrate, sodium phosphate monobasic monohydrate, HTL hydrochloride, creatinine (Crn), tris(2-carboxyethyl)phosphine hydrochloride (TCEP), cysteinyl-glycine (Cys-Gly), symmetrical disulfides of particular aminothiols, namely homocystine (Hcy2), cystine (Cys2) and oxidized glutathione (Glu2), HPLC-gradient grade acetonitrile (ACN), ethanol and methanol, GC-grade chloroform, and acetic acid were from Sigma-Aldrich (St. Louis, MO, USA). Hydrochloric acid and perchloric acid (PCA) were from J.T. Baker (Deventer, Netherlands). 1-Benzyl-2-chloropyridinium bromide (BBCP) and 2-chloro-1-methyllepidinium tetrafluoroborate (CMLT) were prepared in our laboratory as previously described17,18. Ultrapure deionized water was produced in our laboratory.
Instrumentation
In relation to HTL assay, an Agilent 1220 Infinity HPLC system equipped with a binary pump integrated with a two-channel vacuum degasser, autosampler, temperature-controlled column compartment and UV detector (Agilent Technologies, Waldbronn, Germany) was used. Instrument control, data acquisition and analysis were performed using an OpenLAB CDS software. The analyte was separated on ZORBAX SB-C18 (150 × 4.6 mm, 5.0 μm) column from Agilent Technologies (Waldbronn, Germany).
Regarding to the Crn and total Hcy, Cys-Gly, glutathione (Glu) and cysteine (Cys) determination, HPLC analyses were carried out using an Agilent 1220 Infinity HPLC system equipped with a binary pump integrated with two-channel degasser, autosampler, column oven, evaporative light scattering detector (ELSD) 1260 Infinity II series and UV detector. Instrument control, data acquisition and analysis were carried out using OpenLAB CDS ChemStation software. Crn was separated on Poroshell 120 SB-C18 (75 × 4.6 mm, 2.7 μm) column from Agilent Technologies (Waldbronn, Germany), while Hcy, Cys-Gly, Glu and Cys were separated on Aeris PEPTIDE XB-C18 (150 × 4.6 mm, 3.6 μm) column from Phenomenex (Torrance, CA, USA).
For sample shaking, MSC-100 thermo-shaker (Allsheng Instruments Co., Ltd., Hangzhou, China) and Multi-Speed Vortex MSV-3500 (Biosan, Riga, Latvia) were used. During the study, QBD2 dry-block thermostat (Grant Instruments Ltd., Cambridge, UK), a Mikro 220R centrifuge with fast cool function (Hettich Zentrifugen, Tuttlingen, Germany), and a FiveEasy F-20 pH-meter (Mettler Toledo, Greifensee, Switzerland) were also used. For absorbance measurements, the UV-1900 spectrophotometer with the UVProbe software (Shimadzu, Kyoto, Japan) was employed. Samples were stored in an ultra-low-temperature freezer (Panasonic Healthcare Co., Ltd., Sakata, Japan). Water was purified using a Direct-Q 3 UV water purification system (Millipore, Vienna, Austria).
Stock solutions
All solutions were prepared with the use of ultrapure deionized water.
The stock solutions of 0.1 mol/L HTL, Cys2, Hcy2, Glu2, Cys-Gly were prepared by dissolving an appropriate amount of solid substance in hydrochloric acid (0.5 mol/L), while stock solution of 0.15 mol/L Crn was prepared in water. The solutions were kept at 4 °C for no longer than 7 days without noticeable change in their content as recommended in the literature8,19,20. The solutions were stored in polypropylene (PP) microcentrifuge tubes. Their working solutions were prepared daily by diluting a standard solution with water as needed and processed without delay.
The stock solution of 0.1 mol/L BBCP and 0.1 mol/L CMLT were prepared by dissolving an appropriate amount of substance in water. The solution of BBCP was stored in tight glass flasks at 4 °C for no longer than 1 month (Figure S1 a). The working solution of 4.0 mmol/L BBCP was prepared daily by diluting a standard solution with 0.2 mol/L phosphate buffer (PBS) at pH 11.0 as needed and processed without delay (Figure S1 b).
The stock solution of 0.2 mol/L PBS buffer at pH 11.0 and pH 7.8 were prepared by dissolving an appropriate amount of trisodium phosphate in water, and then adjusting the pH with an appropriate volume of 0.2 mol/L water solution of sodium phosphate monobasic. The PBS buffers were prepared once a week and stored in tight glass bottle at room temperature.
The stock solution of 0.2 mol/L sodium phosphate dibasic (pH ~ 8.8) was prepared by dissolving an appropriate amount of solid in water. The solution was stored in tight glass flasks at ambient temperature for no longer 1 week.
The stock solution of TCEP (0.25 mol/L) was prepared by dissolving an appropriate amount of TCEP powder in deionized water as needed and processed without delay. The solution was stored in PP tube at room temperature.
The mobile phase component consisting of 0.1% acetic acid or 0.5% acetic acid was prepared by diluting glacial acetic acid with water. The solution was prepared once a week and stored in tight amber glass bottle at room temperature.
The other solutions, including PCA (3.0 mol/L) and hydrochloric acid (0.5 mol/L, 0.05 mol/L) were prepared by dilution of a particular standard solution with water. All of these solutions were stored in tight glass flasks at ambient temperature for no longer than 1 month.
Biological samples collection
First, early morning urine samples (about 5 mL) were collected from individuals after overnight fasting using standard method21. Samples of urine “mid-stream” were obtained by asking donors to put fluid into a sterile container. Then, samples were cooled on ice and delivered to the laboratory within 3 h after collection. Samples were processed without delay using the procedures described in Sect. 2.5 and Sect. 2.6. In addition, obtained results for urinary HTL content were adjusted for Crn which was assayed by our HPLC-ELSD method19. The urine samples were also analyzed for total Hcy, Cys-Gly, Glu and Cys by our HPLC-UV method20.
A group of eighteen apparently healthy anonymous individuals was involved in the study. The control subjects, belonging to an ethnically homogeneous group, were neither supplemented with the analyte before sample collection, or its precursors. In addition, no medications were allowed. All subjects involved in the study have also declared that, to the best of their knowledge, none of them suffer from any disease.
Urine sample preparation
The urine sample was partially prepared according to previously published and slightly modified method8. Urine (250 µL) was mixed with 125 µL of 0.2 mol/L sodium phosphate dibasic in 2 mL PP microtube. In the next step, the analyte was extracted by shaking using microtube shaking incubator at 1500 rpm for 1 min at 25 °C with 1500 µL of chloroform. Thereafter, the resulting mixture was centrifuged (12,000g, 25 °C, 1 min) and the bottom layer (1300 µL) was transferred into a 2 mL PP microtube. Then, 120 µL of 0.05 mol/L hydrochloric acid was added to the mixture. After shaking at 1500 rpm for 3 min at 25 °C, the upper layer (100 µL) was transferred to a first hydrolytic class glass vial and dried in dry-block thermostat (110 °C, ~ 15 min). After cooling down (~ 5 min), the residue was treated with 50 µL of 4.0 mmol/L BBCP in 0.2 mol/L PBS buffer, pH 11.0. After vortexing at 1500 rpm for 15 min at 25 °C, the reaction mixture was treated with 10 µL of 3.0 mol/L PCA. A 10 µL aliquot of the resulting solution was injected onto reversed phase (RP) column. Each sample was analyzed according to the procedure described in Sect. 2.6.
Chromatographic conditions
Urine samples were analyzed using slightly modified method22,23,24,25. The chromatographic separations were performed on ZORBAX SB-C18 (150 × 4.6 mm, 5.0 μm) column at room temperature using linear gradient elution (0–3.5 min, 10–50% B; 3.5–5.0 min, 50 − 10% B; 5.0–6.0 min, 10% B) with the mobile phase consisted of 0.1% acetic acid (solvent A) and ACN (solvent B), delivered at the flow rate of 1 mL/min. The column was re-equilibrated between analyses by setting the post-run conditioning at 10% B for 0.5 min. The effluent was monitored with UV detector at 316 nm with the bandwidth of 4 nm using 390 nm ± 20 nm as a reference wavelength.
Results and discussion
The article presents the first HPLC-UV based method that allows quantification of urinary HTL. The following (sub)sections of the report provide all necessary information on the development, validation, and in-study use of the proposed herein assay.
Sample preparation
Sample processing involves two crucial steps, namely separation of HTL from the other urine sample constituents, and derivatization of HTL followed by HPLC-UV analysis. In general, these steps are typical for HTL assays7. Sample preparation procedure primarily involves (1) chloroform LLE of HTL and re-LLE of the analyte with diluted hydrochloric acid to separate HTL from low molecular-weight thiols present in urine26,27, in particular Hcy species; (2) drying sample under elevated temperature to remove an excess of acid; (3) treatment of the residue with BBCP under alkaline conditions followed by sample acidification with PCA to enhance UV detectability and terminate the derivatization reaction, respectively. Then, HTL-delivered Hcy in the form of 2-S-pyridinium derivative (Hcy-BBCP) is quantified by HPLC-UV assay. The presented herein sample preparation procedure was partially based on HPLC-MS/MS and HPLC-FLD assays by Piechocka and Głowacki8. We have found out that some characteristics of the above-mentioned approach can be adopted in terms of urine samples purification.
Preliminary considerations
The normal urine pH range is between 4.5 and 8.0, while the most common value for urine pH is 5.5–7.0according to the American Association for Clinical Chemistry. Because it was established by Piechocka and Głowacki8that the efficiency of HTL extraction is dependent on medium pH, a pH adjustment of the sample has become required to minimize its contribution to the recovery losses in this method. As demonstrated previously8, sample pH greater than 7.0 were of analytical value given that dissociation constant of HTL equals to 6.66 7,8. In order to counteract excessive sample dilution, 0.2 mol/L sodium phosphate dibasic was chosen to adjust the sample pH10,11,12. It was found that minimum ratio of 2:1 by volumes, urine to 0.2 mol/L sodium phosphate dibasic is needed to gain a pH for the mixture in the range of 7.1–7.9, regardless of its source and pH of normal urine sample ranging from 4.5 to 7.0. As it has been described in the next sections of the paper, the best results were achieved when 250 µL of urine was mixed with 125 µL of 0.2 mol/L sodium phosphate dibasic and subjected to LLE step without delay due to limited stability of HTL under even slightly alkaline conditions7,8. The LLE and re-LLE processes were proceeded with chloroform (1500 µL) and diluted hydrochloric acid (0.05 mol/L, 120 µL) respectively, as previously described8. The concentration of hydrochloric acid tested in the 0.005–0.1 mol/L range, did not affect operational efficiency.
Interestingly, in an attempt to simplify the sample preparation process, some efforts have been made toward integration of re-LLE and derivatization steps. For this purpose, HTL was re-LLE from chloroform with 4.0 mmol/L BBCP in 0.2 mol/L PBS buffer, pH 11.0 by vortexing at 1500 rpm for 15 min at 25 °C as described in Sect. 2.5. Unfortunately, the outcome of the process was disappointing. It has been recognized that BBCP is transferred from aqueous layer to organic phase. As little as 1.17% of initial concentration of the reagent was found in derivatization mixture after 15 min vortexing indicating that re-LLE has to be performed with 0.05 mol/L hydrochloric acid.
Sample enrichment
In order to counteract sample dilution, extracts (100 µL) were dried before the subsequent chemical derivatization step. Importantly, the removal of the excess of hydrochloric acid from the samples was found to be essential taking into account Le Chatelier’s principle. Hydrochloric acid is a product from chemical reaction of HTL-delivered Hcy and BBCP (Fig. 1 b), and its excess could affect the outcome of the derivatization reaction. During the study, it has been recognized that the registered Hcy-BBCP peak area was approximately 10-times lower when even trace amounts of hydrochloric acid were present in the sample in comparison with reference values. In the presented study, extracts were thus placed in first hydrolytic class glass vials as recommended elsewhere28, and evaporated using dry-block thermostat. Then, the impact of evaporation temperature and time on the analyte-delivered signal intensity was evaluated. The reduction of time from 15 to 6 min, needed for solvent removal from samples, was observed in parallel with temperature rise from 110 to 130 °C. In addition, it has been recognized that duration of the process, tested up to 40 min, affected operational efficiency (Figure S2 a, Figure S2 b, Figure S2 c) regardless of excellent stability of HTL in the form of hydrochloride1,7. The most satisfactory results were obtained when extracts were placed in dry-block thermostat set to 110 °C for at least 15 min and no longer than 20 min (Figure S2 a). Under set conditions, no more than 4.80% of the analyte was lost if compared with reference values.
Derivatization
The presented HPLC-UV method is based on the simultaneous decomposition of HTL and derivatization of HTL-delivered Hcy with the thiol-specific reagent, possessing an active halogen atom as a reactive moiety, namely BBCP. In the case of HTL, derivatization might be considered useless at first glance as it is a UV-absorbing molecule1,7,12,13,14,29. Based on through investigation of literature7, we have nonetheless concluded that chemical modification of the analyte is needed for enhancing UV detectability of HTL and improving its chromatographic behavior in RP-HPLC mode of separation.
Thus far, BBCP has been demonstrated to be an effective derivatizing agent when determining captopril17,30, N-acetylcysteine (NAC)30, Cys31,32,33, Cys-Gly31,32,33, Glu32,33, Hcy32,33, lipoic acid (LA)22,23,33,34and lipoyllysine (LLys)24,25and total thiols concentration35in pharmaceutical formulations30, human plasma17,23,32,33, urine22,24,31,34,35and animal meat25by HPLC-UV17,22,23,24,25,30,31,32,33, CE with UV detection34and spectrophotometry35. It has been shown that BBCP reacts efficiently in 5–15 min at room temperature with the above mentioned sulfur-containing compounds in alkaline solutions (pH for the reaction mixture in the range of 7.0–11.0) to give corresponding UV-absorbing 2-S-pyridinium derivatives, exhibiting absorption maximum wavelength at around 314–321 nm.
During the study, it has been found out that derivatization of HTL with BBCP also occurs under alkaline conditions, but it requires longer reaction time. The reaction results in production of UV-absorbing Hcy-BBCP derivative. It is also analytically important that the reaction is accompanied by bathochromic shift of absorption bands of the derivative compared with its precursors (Fig. 2 a, Fig. 2 b). In fact, it is a multi-step process in which the instability of the analyte under extremely alkaline conditions (pH ≥ 11.0) is of key importance1,7. Based on literature data1,7,32, it has been concluded that HTL firstly undergoes an equilibrium ring opening resulting in Hcy release. Simultaneous with that process, halide leaving group of BBCP is displaced by highly nucleophilic thiol group of Hcy, and it leads to formation of a HTL-delivered Hcy-BBCP adduct and hydrochloric acid release (Fig. 1 a, Fig. 1 b). Thus far, the HTL susceptibility to alkaline hydrolysis has been used in methods employing OPA to form fluorescent derivative9,10,11,36,37,38. As the presented assay utilizes the limited stability of HTL under extremely alkaline conditions at the stage of derivatization with BBCP for the first time, through studies have been thus performed in order to optimize the process.
(a) UV absorption spectrum of Hcy-BBCP derivative (solid line) and BBCP (dashed line) obtained by analyzing quality control sample assayed according to procedures described in Sect. 2.5 and Sect. 2.6. (b) UV absorption spectrum of BBCP obtained by analyzing its standard solution prepared in water (solid line) and 0.2 mol/L PBS buffer, pH 11.0 (dashed line) assayed using UV-1900 spectrophotometer.
In the beginning, the impact of the pH of the reaction medium on process efficiency was studied within the range of 11.0–13.0. In order to control the pH level, 0.2 mol/L PBS buffer was selected having high capacity and effective buffering range in the defined above regions of pH. It was recognized that the rate of HTL decomposition was dependent on the pH, but it did not have an impact on the high yields of the derivatization process (Figure S3). From the purely practical point of view, 0.2 mol/L PBS buffer at pH 11.0 was chosen as it was easier to control the course of the reaction and obtain reproducible results. For further experiments, the minimal volume of the solution (50 µL) was used to cover the extract residue and bottom of the vial in order to prevent excessive sample dilution.
Subsequently, other factors having an impact on derivatization reaction yield have been optimized, namely the quantity of BBCP, temperature and reaction time. It was found that temperature, tested in the range of 20–30 °C, does not effect it. Thus, the reaction course was examined at room temperature in the range of 0–180 min by applying BBCP solutions at different concentration levels, varied from 2 to 400 mmol/L, providing the concentration of BBCP in reaction mixture in the range of 0.09–18.18 mmol/L. At the same time, the stability of Hcy-BBCP derivative was assessed. The increase of the analyte-delivered peak area was observed in parallel with the concentration of BBCP rise from 0.09 to 1.82 mmol/L, and then there were generally no significant changes (Figure S4 a). Interestingly, we have also recognized that higher analyte recovery, better reproducibility and replicability of the process were achieved when samples were shaken instead of put aside. The progressive rise of the signal peak area was observed concomitant with shaking frequency rise from 300 rpm to the highest possible shaking frequency of 1500 rpm (Figure S4 c). Finally, for routine analysis, the extract residue was treated with 50 µL of 4.0 mmol/L BBCP in 0.2 mol/L PBS buffer, pH 11.0 and placed in vortex shaker set to 1500 rpm. A larger excess of BBCP was excluded as it was the source of additional baseline noises. Under set conditions, the reaction was finished in 15 min at ambient temperature with a high level of precision (Figure S4 b). An additional advantage was also good stability of the Hcy-BBCP derivative under alkaline conditions. As shown in Figure S4 b, it remains stable for the next 30 min making possible to prepare multiple samples not under time regime. Nevertheless, it is recommended to acidify samples after completion of the derivatization reaction to prolong stability of the obtained product.
During the study, the stability of stock and working solutions of BBCP was also determined as we found that such data are not available in the literature. The stability of the reagent and derivatizing mixture was evaluated at 25 °C and 4 °C. In the stability experiments of stock solution of BBCP and derivatizing mixture were prepared in preselected time intervals across 1 month and assayed under described herein HPLC-UV conditions. In order to assess the total time that a processed sample can be treated with the derivatizing mixture, the solution containing 4.0 mmol/L BBCP in 0.2 mol/L PBS buffer, pH 11.0 was analyzed across one working day. It was recognized that the content of BBCP remains stable at any examined conditions in the tested period of time since a drop to 98.79% (Figure S1 a) and 99.63% (Figure S1 b) initial concentration was observed in relation to stock and working solutions of BBCP, respectively. It has been thus concluded that the stock solution of BBCP and derivatizing mixture can be prepared once per month and day, respectively without noticeable change in its content.
Sample acidification
According to literature data17,23,25,32, 2-S-pyridinium derivatives of some low molecular-weight sulfur-containing compounds, including Hcy-BBCP derivative32, are stable under acidic conditions. In order to prolong stability of the obtained product, samples were acidified with 3.0 mol/L PCA as reported elsewhere22,23,24,25,31,32,34,35. The process was also essential to terminate the derivatization reaction. Samples were acidified by mixing the sample with 3.0 mol/L PCA and minimum crashing at 5:1 ratio by volumes.
Stability of processed samples
The total time and conditions that a processed samples are kept until completion of analysis were evaluated as recommended in the literature39. The stability of the Hcy-BBCP derivative was assessed at 25 °C and 4 °C. It was recognized that the sample remains stable at least 1 month, regardless the concentration of the analyte within the quantitation range and examined conditions. When study samples were left at 25 °C and 4 °C, the drop to 99.33% and 99.64% of the initial concentration was observed, respectively (Figure S5 a, Figure S5 b). The freeze-thaw stability test of processed samples has also shown that reliable and valid HPLC-UV data can be obtained when even five times thawed samples are analyzed (Figure S6) as the recovery of 99.88% was observed. These results agree with the literature data and support information that 2-S-pyridinium derivative of Cys, Glu, Hcy, Cys-Gly, LA, LLys and captopril are highly stable compounds under acidic conditions17,23,25,32. The Hcy-BBCP derivative stability make possible to prepare a large batch of samples.
In overall, the research demonstrates that BBCP is suitable derivatizing agent for not only captopril17, Cys31,32,33, Cys-Gly31,32,33, Glu32,33, Hcy32,33, LA22,23,33,34and LLys24,25, but also HTL. Regarding HTL, this is a first piece of data on this topic to the best of our knowledge. Moreover, human urine samples preparation procedure for HTL determination by HPLC-UV assay has been elaborated. It is also without analytical importance that Hcy-BBCP derivative exhibits an excellent stability under experimental conditions allowing to prepare a large batch of samples which contributes to the effort minimization. On the other hand, a serious limitation of the presented method is multistep sample preparation procedure. Overall sample preparation time of a set of twenty four samples was estimated to be around 60 min (~ 40 min per sample) (Fig. 3). The time of sample pretreatment is nevertheless longer in comparison with the latest HPLC-MS/MS and HPLC-FLD based method that requires no more than 15 min8. In relation to other urinary HTL assays, sample preparation time is comparable or even shorter7. Regarding to the presented method, the process consumes of 1805 µL of inexpensive chemicals per 250 µL sample and it is quite high when comparing to other assays. Detailed information on this topic can be found here7.
Chromatographic and detection conditions
The presented herein urinary HTL assay involves separation of the HTL-delivered Hcy and BBCP derivative with the use of RP-HPLC mode of separation followed by UV detection. A standard approach was employed to find optimal HPLC-UV conditions which provides the best method`s performance.
Selection of separation conditions
Thus far, numerous methods for the determination of some low molecular-weight sulfur-containing compounds in the form of 2-S-pyridinium derivatives have been elaborated17,22,23,24,25,30,31,32,33,34,35. In particular, it has been demonstrated that RP-HPLC coupled with UV detection is suitable for this purpose17,22,23,24,25,30,31,32,33. In these methods, the chromatographic separations were accomplished at room temperature22,23,24,25,31,32,33or elevated temperature (45–50 °C)17,30using isocratic17,30or linear gradient elution22,23,24,25,31,32,33 under acidic conditions (pH for the mobile phase in the range of 2.2–2.6). The corresponding 2-S-pyridinium derivatives were eluted with the mobile phase consisted of acetone17,30or ACN22,23,24,25,31,32,33, used as an organic mobile phase modifier, and 0.2 mol/L citrate buffer17,30, 0.1 mol/L trichloroacetic acid31,32,33or 2% acetic acid22,23,24,25. In addition, it has been reported that an initial organic mobile phase modifier content no higher than 10% is essential to retain target compounds providing that chromatographic separations are performed with eluent at acidic pH, delivered at the flow rate of 1 mL/min at ambient temperature17,22,23,24,25,30,31,32,33.
In the present study, initial experiments were performed according to the adopted approaches22,23,24,25using ZORBAX SB-C18 column for RP separations as recommended elsewhere22,23,24,25,31,32,33. In fact, efficient resolution of Hcy-BBCP from the other sample components as well as proper equilibration of the chromatographic system between analyses in a relatively short analysis time were achieved by means of modifying run gradient profile as well as selecting a mixture of ACN and water with as little as 0.1% acetic acid (pH ~ 3.2) as mobile phase to decrease the eluent components environmental impacts. Under set conditions, the analyte was eluted within 3 min and its peak was easy to differentiate from the other responses on chromatogram (Fig. 4 and Figure S7). Each time the elution profile of blank samples was free from any interference at the retention time of the analyte. As these results were satisfactory in terms of retention of the compound of interest, its peak shape and resolution, separation conditions were not furtherly optimized. In general, the analysis time 6 min is longer than in other urinary HTL assays such as HPLC-FLD9,10, GC-MS15and CE-UV12where it is 3–5 min. In some cases, the duration of HPLC analysis is comparable8or even more favorable than in other HPLC-FLD11, GC-MS16and CE-UV13,14based methods, where it is 12 min11, 15 min16and 7–10 min13,14, respectively.
Representative chromatograms of standard solutions and human urine prepared according to the procedure described in Sect. 2.5. Chromatographic conditions were as described in Sect. 2.6. Blank standard solution (black dashed line), blank human urine (grey line) and the same sample spiked with HTL (1 µmol/L in urine) (black solid line). Under these conditions, the analyte-delivered signal appears at 2.63 min.
Selection of detection conditions
During the study, it has been recognized that reaction of HTL with BBCP results in production of UV-absorbing Hcy-BBCP derivative, exhibiting absorption peak near 316 nm (Fig. 2 a). The target compound was thus quantified using 316 nm as it also allow to increase sensitivity and selectivity in trace analysis.
The identification and confirmation of the analyte delivered peak were conducted by analyzing the standard solution of HTL prepared and analyzed according to the procedures described in Sect. 2.5 and Sect. 2.6. Moreover, the Hcy-BBCP peak was evaluated for purity. For this purpose, the analyses were carried out with HPLC system coupled with UV detector set to collect time and spectral information throughout the entire chromatogram. Importantly, the same spectra were collected in different sections of the particular analyte peak, indicating its purity. The analyte-delivered peak disappearance after base hydrolysis before applying sample to the sample preparation procedure and HPLC analysis was also helpful in confirming HTL identity since the analyte is susceptible to hydrolysis1,7.
Finally, the confirmation of the origin of analyte-delivered peak and quantification of HTL in urine samples were made by comparing of retention time and UV spectrum with the set of data obtained by analyzing an analytical standard of HTL.
Greenness assessment of the method
An AGREE - Analytical GREEnness tool (version 0.5 beta)40 was used to identify strong and weak points of the delivered method in terms of its environmental friendliness. Equal weights were set for all evaluated parameters. The below indicated assumptions have been made: procedure involves an external sample (pre)treatment with a reduced number of steps (principle 1); the volume of urine is 0.25 mL (principle 2); the analytical device is positioned off-line (principle 3); the number of distinctive analytical steps is five, including LLE and re-LLE of HTL, sample evaporation, chemical derivatization and sample acidification followed by HPLC-UV analysis (principle 4); the procedure is semi-automated and involves a miniaturized sample preparation methods (principle 5); derivatization step is required (principle 6); the total amount of waste is 18.16 (g and mL combined), including sample itself, chemicals, and plastic disposable ware (principle 7); one analyte is determined in a single run, and the sample throughput is ten samples per hour (principle 8); the most demanding technique is HPLC-UV (principle 9); none of the reagents can be obtained from bio-based sources (principle 10); the procedure requires approximately 3.60 mL of toxic chemical reagents, including ACN and chloroform (principle 11); ACN is harmful and highly flammable, while chloroform is toxic and health hazard (principle 12). Acetic acid, PCA, and hydrochloric acid were not taken into account at the stage of assessing the operator’s safety since their trace amounts were used. The overall score is 0.46 (Fig. 5).
As shown in Fig. 5, the attractiveness of the presented method relies on a opportunity of performing analysis on a small scale. Nevertheless, the process is accompanied by relatively high consumption of chemicals and laboratory disposable plastic ware. In addition, semi-automation of the analytical procedures allows for reduction of labor-intensity, maximization of sample throughput, improving accuracy and reproducibility of the assay. On the other hand, a serious limitation of the presented method is the need of performing analyses with the use of energy-intensive measurement technique and toxic chemicals. Simple but multistep sample preparation procedure as well as relatively low sample throughput potential represent an additional limitations. In overall, the presented herein assay falls into some principles of Green Analytical Chemistry40.
Validation of the method
Full validation of the HPLC-UV method was conducted. Studies were conducted in accordance with the latest International Council for Harmonization harmonized guideline for bioanalytical methods validation39. The same protocol was employed as described in detail in our previously published article concerning HPLC-MS/MS and HPLC-FLD assays for the analysis of urinary HTL8. The process involved assessment of selectivity and specificity, linearity, intra-/inter-assay precision and accuracy, limit of quantification (LOQ), matrix effect, carry-over, and reinjection reproducibility. Some parameters which can be checked among system suitability testing were also evaluated. The experiments were made using freshly laboratory-made pooled blank urine samples which were spiked with known quantities of HTL to prepare calibration standards.
System suitability
System suitability parameters including repeatability of chromatographic retention / peak area of the analyte, expressed as the coefficient of variation (CV) of retention time / peak area, symmetry factor, and number of theoretical plates were nominated during the method validation to determine instrument performance under optimized conditions. System suitability test calculations were performed as a part of linearity evaluation, and the calibration standards at the upper LOQ (ULOQ) were analyzed in ten replicate injections. The CV value of retention time and peak area was 0.095% and 0.22% respectively (acceptance criteria ≤ 1%), the mean symmetry factor was 1.06 (acceptance criteria 0.8–1.5), and number of theoretical plates was 16 229 (acceptance criteria ≥ 2000) for the analyte assayed by HPLC-UV method.
Selectivity and specificity
The selectivity of the method was assessed at first attempt during studies concerning the identification and confirmation of the origin of HTL-delivered peak as described in Sect. 3.2. Selectivity and specificity studies evaluated interferences originating from all (un)known components of study samples, in particular Cys, Glu, Hcy, Cys-Gly, LA, LLys and NAC which are present in normal urine specimens20,22,24,27,31,34 and known to give corresponding UV-absorbing 2-S-pyridinium derivatives17,22,23,24,25,30,31,32,33,34. Importantly, the studies evaluated interferences originating from Hcy being the precursor/degradation product of HTL, although Hcy is not (re)-LLE under set conditions7. Several types of samples were assayed, including (1) blank standard solution, (2) standard solution of reduced/oxidized form of Cys, Glu, Hcy, Cys-Gly, LA, LLys, NAC, and HTL (if applicable), (3) blank urine samples from six control and (4) the same blank urine samples spiked with reduced/oxidized form of Cys, Glu, Hcy, Cys-Gly, LA, LLys, NAC, and HTL (if applicable). As shown in Fig. 4 and Figure S7, no significant response attributable to interfering components was observed at the retention time of Hcy-BBCP in the blank samples. Under these conditions, the reduced/oxidized form of Cys, Glu, Hcy, Cys-Gly, LA, LLys and NAC (if applicable) are not (re)-LLE under experimental conditions. In order to provide definite proof that the HPLC-UV assay can discriminate HTL in a complex mixture without interference from other sample components, a blank standard solution and a standard solution of reduced/oxidized form of Cys-Gly, Cys, Hcy, Glu, NAC, LLys and LA (if applicable) was assayed without applying sample, prepared according to the procedure described in Sect. 2.5, to the LLE and re-LLE processes. Under these conditions, the LA, LLys and oxidized form of Cys-Gly, Cys, Hcy, Glu, and NAC do not react with BBCP, while their reduced forms give a corresponding 2-S-pyridinium derivative eluting at 2.24 min, 2.34 min, 2.63 min, 2.89 min, 3.45 min, respectively (Figure S7). The order of elution was as reported elsewhere24. Peak purity tests also confirmed the ability of HPLC-UV method to differentiate and measure HTL in the presence of matrix components in urine samples.
Linearity
An external standard addition method was used to test the calibration range of 0.1–1µmol/L in urine. Calibration curves consisting of blank sample and five calibrators at the level of 0.10, 0.25, 0.50, 0.75, 1.0 µmol/L were generated and were run in triplicate over 3 days. The concentrations of HTL in calibration standards were chosen taking into account the concentration range expected in real urine samples7. The concentration-response relationship (Hcy-BBCP peak area) was described using the least-squares regression model. The calibration standards fitted well into the linear regime within the intended quantification range, which gave correlation coefficient values of 0.9980. Substantial changes in the slope of any regression line obtained over 3 subsequent days were not detected denoting the absence of matrix effect. Detailed data on validation parameters, which were evaluated during the linearity assessment, are presented in Table 1.
Precision and accuracy
The intra- and inter-day accuracy and precision of the assay were assessed during linearity evaluation. The precision was expressed as CV of measurement repeatability. Accuracy was shown as the percentage of analyte recovery. The accuracy was calculated by expressing the mean measured amount as a percentage of added amount of HTL (nominal concentration). The accuracy varied from 92.56 to 112.51% and 92.56–114.31% for intra- and inter-day measurements, respectively. In parallel, the precision ranged from 5.89 to 11.27% and 3.85–14.25% for intra- and inter-day variations, respectively. Detailed data on precision and accuracy from the 3 day experiments are shown in Table 2.
The limit of quantification
The LOQ was calculated considering the parameters of the analytical curves performed for urine samples from six individual sources, pooled matrix and surrogate matrix (0.2 mol/L PBS buffer, pH 7.4) with the use of equation: LOQ = 10 × SDa / b, where SDais the standard deviation of the y-intercept of the calibration curve and b is the slope of the regression line. In addition, the LOQ was determined experimentally by the signal-to-noise method. For this purpose, a surrogate matrix was spiked with decreasing concentrations of HTL and samples were then handled according to the procedures described in Sect. 2.5 and Sect. 2.6 until the injected amount resulted in a peak 10-times as high as the baseline signal. The surrogate matrix was employed to assess the LOQ as the assay is not affected by matrix effects. The estimated LOQ value using two independent methods closely corresponded to each other and equals to 100 nmol/L in urine. As demonstrated upon validation, this concentration of HTL produced reproducible detector response with a precision and accuracy of 11.27% and 92.56%, respectively. The accepted LOQ value was similar or even better than that reported earlier concerning determination of urinary HTL by CE-UV12,14and HPLC-FLD assays10,11or higher than that obtained by GC-MS15,16, HPLC-FLD9, CE-UV13, and HPLC-MS/MS methods8. However, the estimated LOQ value make possible the determination of HTL in human urine.
Carry-over effect
Carry-over evaluation was performed during the linearity assessment. For this purpose, blank standard solution samples were analyzed after the calibration standard at the ULOQ. Every time the response of blank samples was as high as background signal. This observation denoted the absence of carry-over effect. The registered chromatograms during carry-over effect evaluation were similar to the elution profile of blank samples presented in Fig. 4 and Figure S7.
Matrix effect
The matrix effect was assessed in a relevant population referring to a group of apparently healthy volunteers enrolled in the study. The matrix effect evaluation involved comparing calibration curves of the six individual sources of urine samples against a calibration curve of the pooled matrix and surrogate matrix (0.2 mol/L PBS buffer, pH 7.4). The CV of the slope of the regression lines did not deviate by more than 4.03% indicating the absence of any matrix effect. Since interpatient variability was not detected, an external standard addition method was employed to quantify the content of HTL in study samples.
Reinjection reproducibility
Reinjection reproducibility test calculations were conducted using the obtained data during evaluation of stability of processed samples since their excellent stability was demonstrated. In this way, the evaluation of reinjection reproducibility was conducted by analyzing the calibration standard(s) at the lower LOQ and ULOQ after keeping them at autosampler temperature (25 °C and 4 °C). In total, the samples were injected in 13 replicates across 1 month. Reinjection repeatability was expressed as CV of the Hcy-BBCP peak area. The CV value of the parameter did not exceed 0.67% demonstrating that reinjection of study samples can be made within 1 month as indicated during stability testing.
All in all, it has been demonstrated upon validation that all assessed validation parameters fall within acceptable tolerance limits39 indicating that the performance of the new method is satisfactory. Based on the obtained results it was concluded that the presented method can be used in diagnostic testing of urine samples for HTL.
Application of the method
The HPLC-UV assay was used to determine HTL in human urine samples from presumably healthy donors. External standard addition method was used to establish the concentration of HTL in study samples. The content of HTL in urine samples was calculated using the mathematical formula corresponding to the equation of the particular calibration line generated on that occasion. Moreover, the obtained results for urinary HTL content were adjusted for Crn. In order to facilitate interpretation of the obtained results, urine samples were also assayed to determine total Hcy, Cys-Gly, Glu and Cys content. HTL was present in twelve of eighteen urine samples delivered by anonymous individuals. The estimated concentrations of HTL, based on data obtained by triplicate analysis of a particular sample from an individual source, varied from 0.0367 to 0.6931 µmol/L in urine (2.08–85.13 µmol/mol Crn) (Table 3). Importantly, these values were similar to those previously reported8,9,11,12,15,16, denoting the data’s reliability from the presented HPLC-UV assay.
Conclusions
The paper presents the first HPLC-UV method for quantification of HTL in human urine. Furthermore, the report provides evidence that the practical application of BBCP in sulfur-containing compounds analysis can be extended to intramolecular thioesters. The presented herein method`s performance allows its use in diagnostic testing of urine samples for HTL. The use of common equipment in worldwide laboratories, which is known for its stability and low demand in terms of maintenance, represents an additional advantage. The presented method should facilitate studies exploring the role of HTL in health and disease.
Data availability
Essential data is contained within the article and supplementary materials file. In addition, the dataset generated and analyzed during this study, which contributed to the article, can be made available by the corresponding author upon reasonable request as long as the request does not compromise intellectual property interests. Urine samples are not available from the authors.
Abbreviations
- ACN:
-
acetonitrile
- BBCP:
-
1-benzyl-2-chloropyridinium bromide
- CE:
-
capillary electrophoresis
- CMLT:
-
2-chloro-1-methyllepidinium tetrafluoroborate
- Crn:
-
creatinine
- Cys:
-
cysteine
- Cys2 :
-
cystine
- Cys-Gly:
-
cysteinyl-glycine
- CV:
-
coefficient of variation
- ELSD:
-
light scattering detector
- FLD:
-
fluorescence detector
- GC:
-
gas chromatography
- Glu:
-
glutathione
- Glu2 :
-
oxidized glutathione
- Hcy:
-
homocysteine
- Hcy-BBCP:
-
2-S-pyridinium derivative of homocysteine
- Hcy2 :
-
homocystine
- HPLC:
-
high performance liquid chromatography
- HTL:
-
homocysteine thiolactone
- LA:
-
lipoic acid
- LLE:
-
liquid-liquid extraction
- LLys:
-
lipoyllysine
- (MS)/MS:
-
(tandem) mass spectrometry
- NAC:
-
N-acetylcysteine
- OPA:
-
o-phthaldialdehyde
- PBS:
-
phosphate buffer
- PCA:
-
perchloric acid
- PP:
-
polypropylene
- RP:
-
reversed phase
- TCEP:
-
tris(2-carboxyethyl)phosphine hydrochloride
- ULOQ:
-
upper limit of quantification
- UV:
-
ultraviolet detection
References
Jakubowski, H. & Głowacki, R. Chemical biology of homocysteine Thiolactone and related metabolites. Adv. Clin. Chem. 55, 81–103 (2011).
Jakubowski, H. Homocysteine in Protein Structure/Function and Human Disease. Chemical Biology of Homocysteine-Containing Proteins (Springer Vienna, 2013).
Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 99, 555–604 (2019).
Perła-Kaján, J., Twardowski, T. & Jakubowski, H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 32, 561–572 (2007).
Borowczyk, K. et al. Urinary excretion of homocysteine Thiolactone and the risk of acute myocardial infarction in coronary artery disease patients: the WENBIT trial. J. Intern. Med. 285, 232–244 (2019).
Sikora, M. et al. Homocysteine Thiolactone and other sulfur-containing amino acid metabolites are associated with fibrin clot properties and the risk of ischemic stroke. Sci. Rep. 14, 11222, 1–13 (2024).
Piechocka, J. & Głowacki, R. Up-to-date knowledge about analytical methods for homocysteine Thiolactone determination in biological samples. TrAC - Trends Anal. Chem. 159, 116906, 1–15 (2023).
Piechocka, J. & Głowacki, R. Comprehensive studies on the development of HPLC-MS/MS and HPLC-FL based methods for routine determination of homocysteine Thiolactone in human urine. Talanta 272, 125791, 1–13 (2024).
Głowacki, R., Bald, E. & Jakubowski, H. An on-column derivatization method for the determination of homocysteine-thiolactone and protein N-linked homocysteine. Amino Acids. 41, 187–194 (2011).
Jakubowski, H. Quantification of urinary S- and N-homocysteinylated protein and homocysteine-thiolactone in mice. Anal. Biochem. 508, 118–123 (2016).
Chwatko, G. & Jakubowski, H. Urinary excretion of homocysteine-thiolactone in humans. Clin. Chem. 51, 408–415 (2005).
Furmaniak, P., Kubalczyk, P. & Głowacki, R. Determination of homocysteine Thiolactone in urine by field amplified sample injection and sweeping MEKC method with UV detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 961, 36–41 (2014).
Purgat, K., Olejarz, P., Kośka, I., Głowacki, R. & Kubalczyk, P. Determination of homocysteine Thiolactone in human urine by capillary zone electrophoresis and single drop Microextraction. Anal. Biochem. 596, 113640, 1–6 (2020).
Purgat, K., Kośka, I. & Kubalczyk, P. The use of single drop Microextraction and field amplified sample injection for CZE determination of homocysteine Thiolactone in urine. Molecules 26, 5687, 1–13 (2021).
Piechocka, J., Wrońska, M., Chwatko, G., Jakubowski, H. & Głowacki, R. Quantification of homocysteine Thiolactone in human saliva and urine by gas chromatography-mass spectrometry. J. Chromatogr. B. 1149, 122155, 1–7 (2020).
Wrońska, M. et al. Application of GC–MS technique for the determination of homocysteine Thiolactone in human urine. J. Chromatogr. B. 1099, 18–24 (2018).
Bald, E., Sypniewski, S., Drzewoski, J. & Stępień, M. Application of 2-halopyridinium salts as ultraviolet derivatization reagents and solid-phase extraction for determination of Captopril in human plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 681, 283–289 (1996).
Głowacki, R., Borowczyk, K. & Bald, E. Determination of Nɛ-homocysteinyl-lysine and γ-glutamylcysteine in plasma by liquid chromatography with UV-detection. J. Anal. Chem. 69, 583–589 (2014).
Piechocka, J. et al. Application of the HPLC-ELSD technique for the determination of major metabolites of ibuprofen and creatinine in human urine. Sci. Rep. 13, 20268, 1–14 (2023).
Stachniuk, J., Kubalczyk, P., Furmaniak, P. & Głowacki, R. A versatile method for analysis of saliva, plasma and urine for total thiols using HPLC with UV detection. Talanta 155, 70–77 (2016).
González-Domínguez, R., González-Domínguez, Á. & Sayago, A. Fernández-Recamales, Á. Recommendations and best practices for standardizing the pre-analytical processing of blood and urine samples in metabolomics. Metabolites 10, 229, 1–18 (2020).
Chwatko, G., Kubalczyk, P. & Bald, E. Determination of lipoic acid in the form of 2-S-pyridinium derivative by high-performance liquid chromatography with ultraviolet detection. Curr. Anal. Chem. 10, 320–325 (2014).
Chwatko, G. et al. Determination of lipoic acid in human plasma by high-performance liquid chromatography with ultraviolet detection. Arab. J. Chem. 12, 4878–4886 (2019).
Kamińska, A., Głowacka, I. E., Pasternak, B., Głowacki, R. & Chwatko, G. The first method for determination of Lipoyllysine in human urine after oral lipoic acid supplementation. Bioanalysis 11, 1359–1373 (2019).
Kamińska, A. & Chwatko, G. Estimation of Lipoyllysine content in meat and its antioxidative capacity. J. Agric. Food Chem. 68, 10992–10999 (2020).
Kuśmierek, K., Chwatko, G., Głowacki, R. & Bald, E. Determination of endogenous thiols and thiol drugs in urine by HPLC with ultraviolet detection. J. Chromatogr. B. 877, 3300–3308 (2009).
Głowacki, R., Piechocka, J., Bald, E. & Chwatko, G. Application of separation techniques in analytics of biologically relevant sulfur compounds. in Handbook of Bioanalytics (eds Buszewski, B. & Baranowska, I.) 233–256 (Springer Nature Switzerland, (2022).
Piechocka, J. & Głowacki, R. One-pot sample Preparation procedure for the determination of protein N-linked homocysteine by HPLC-FLD based method. J. Chromatogr. B. 1228, 123835, 1–7 (2023).
Jakubowski, H. The determination of homocysteine-thiolactone in biological samples. Anal. Biochem. 308, 112–119 (2002).
Bald, E. & Sypniewski, S. Determination of thiol drugs in pharmaceutical formulations as their S-pyridinium derivatives by high-performance liquid chromatography with ultraviolet detection. Fresenius J. Anal. Chem. 358, 554–555 (1997).
Kuśmierek, K. & Bald, E. Analysis of major urinary aminothiols by high-performance liquid chromatography with ultraviolet detection. Acta Chromatogr. 21, 411–420 (2009).
Kuśmierek, K. & Bald, E. Reversed-phase liquid chromatography method for the determination of total plasma thiols after derivatization with 1-benzyl-2-chloropyridinium bromide. Biomed. Chromatogr. 23, 770–775 (2009).
Borowczyk, K., Olejarz, P., Chwatko, G., Szylberg, M. & Głowacki, R. A simplified method for simultaneous determination of α-lipoic acid and low-molecular-mass thiols in human plasma. Int. J. Mol. Sci. 21, 1049, 1–15 (2020).
Kubalczyk, P. & Głowacki, R. Determination of lipoic acid in human urine by capillary zone electrophoresis. Electrophoresis 38, 1800–1805 (2017).
Chwatko, G. Spectrophotometric method for the determination of total thiols in human urine. Ann. Clin. Lab. Sci. 43, 424–428 (2013).
Mukai, Y., Togawa, T., Suzuki, T., Ohata, K. & Tanabe, S. Determination of homocysteine Thiolactone and homocysteine in cell cultures using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B. 767, 263–268 (2002).
Tsiasioti, A., Zacharis, C. K. & Tzanavaras, P. D. Single-step hydrolysis and derivatization of homocysteine Thiolactone using zone fluidics: simultaneous analysis of mixtures with homocysteine following separation by fluorosurfactant-modified gold nanoparticles. Molecules 27 (2040), 1–9 (2022).
Chwatko, G. & Jakubowski, H. The determination of homocysteine-thiolactone in human plasma. Anal. Biochem. 337, 271–277 (2005).
ICH for. M10 Guideline for Bioanalytical Method Validation and Study Sample Analysis. (2022).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE - Analytical greenness metric approach and software. Anal. Chem. 92, 10076–10082 (2020).
Acknowledgements
The authors gratefully acknowledge all the volunteers from the Faculty of Chemistry, University of Lodz for the donation of urine samples. We also gratefully acknowledge Paul Ricque, an Erasmus+ student from ESCOM - School of Organic and Mineral Chemistry, Compiègne, France, for technical assistance.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors acknowledge the financial support in covering the costs from the University of Lodz (B2411100000234.01).
Author information
Authors and Affiliations
Contributions
M.G.: Investigation, Visualization. R.G.: Critical review, Resources, Funding. J.P.: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing – original draft, Writing – review and editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
CRediT authorship contribution statement
M.G.: Investigation, Visualization. R.G.: Critical review, Resources, Funding. J.P.: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing – original draft, Writing – review and editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors did not use generative AI and AI-assisted technologies.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Lodz (decision identification code 2/KEBN-UŁ/V/2022-23, date of approval 2 June 2023).
Informed consent statement
Written informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gaweł, M., Głowacki, R. & Piechocka, J. First HPLC-UV method for the determination of homocysteine thiolactone in human urine after derivatization with 1-benzyl-2-chloropyridinium bromide. Sci Rep 15, 7310 (2025). https://doi.org/10.1038/s41598-025-92323-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92323-y