Abstract
Multidrug-resistant bacteria are spreading at alarming rates, and despite extensive efforts no new class of antibiotic with activity against Gram-negative bacteria has been approved in over fifty years. Natural products and their derivatives have a key role in combating Gram-negative pathogens. Here we report chemical optimization of the arylomycins—a class of natural products with weak activity and limited spectrum—to obtain G0775, a molecule with potent, broad-spectrum activity against Gram-negative bacteria. G0775 inhibits the essential bacterial type I signal peptidase, a new antibiotic target, through an unprecedented molecular mechanism. It circumvents existing antibiotic resistance mechanisms and retains activity against contemporary multidrug-resistant Gram-negative clinical isolates in vitro and in several in vivo infection models. These findings demonstrate that optimized arylomycin analogues such as G0775 could translate into new therapies to address the growing threat of multidrug-resistant Gram-negative infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The sequence reported in this paper has been deposited in the GenBank database, www.ncbi.nlm.nih.gov/genbank (accession numbers CP022611–CP022613). G0775, G3031, G8126 and G6850 are unique molecules proprietary to Genentech that are readily available on request. The authors declare that all other data supporting the findings of this study are available from the authors and/or included with the manuscript as Source Data (efficacy studies) or as Supplementary Information.
References
Ventola, C. L. The antibiotic resistance crisis part 1: causes and threats. P&T 40, 277–283 (2015).
Rice, L. B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197, 1079–1081 (2008).
Schimana, J. et al. Arylomycins A and B, new biaryl-bridged lipopeptide antibiotics produced by Streptomyces sp. Tü 6075. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. (Tokyo) 55, 565–570 (2002).
Kulanthaivel, P. et al. Novel lipoglycopeptides as inhibitors of bacterial signal peptidase I. J. Biol. Chem. 279, 36250–36258 (2004).
Smith, P. A., Powers, M. E., Roberts, T. C. & Romesberg, F. E. In vitro activities of arylomycin natural-product antibiotics against Staphylococcus epidermidis and other coagulase-negative staphylococci. Antimicrob. Agents Chemother. 55, 1130–1134 (2011).
Paetzel, M., Dalbey, R. E. & Strynadka, N. C. The structure and mechanism of bacterial type I signal peptidases. A novel antibiotic target. Pharmacol. Ther. 87, 27–49 (2000).
Smitha, R. C. & Anné, J. Bacterial type I signal peptidases as antibiotic targets. Future Microbiol. 6, 1279–1296 (2011).
Bruton, G. et al. Lipopeptide substrates for SpsB, the Staphylococcus aureus type I signal peptidase: design, conformation and conversion to α-ketoamide inhibitors. Eur. J. Med. Chem. 38, 351–356 (2003).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6, 29–40 (2007).
O’Shea, R. & Moser, H. E. Physicochemical properties of antibacterial compounds: implications for drug discovery. J. Med. Chem. 51, 2871–2878 (2008).
Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003).
Brown, D. G., May-Dracka, T. L., Gagnon, M. M. & Tommasi, R. Trends and exceptions of physical properties on antibacterial activity for Gram-positive and Gram-negative pathogens. J. Med. Chem. 57, 10144–10161 (2014).
Smith, P. A., Roberts, T. C. & Romesberg, F. E. Broad-spectrum antibiotic activity of the arylomycin natural products is masked by natural target mutations. Chem. Biol. 17, 1223–1231 (2010).
Liu, J., Smith, P. A., Steed, D. B. & Romesberg, F. Efforts toward broadening the spectrum of arylomycin antibiotic activity. Bioorg. Med. Chem. Lett. 23, 5654–5659 (2013).
Luo, C., Roussel, P., Dreier, J., Page, M. G. P. & Paetzel, M. Crystallographic analysis of bacterial signal peptidase in ternary complex with arylomycin A2 and a β-sultam inhibitor. Biochemistry 48, 8976–8984 (2009).
Liu, J. et al. Synthesis and characterization of the arylomycin lipoglycopeptide antibiotics and the crystallographic analysis of their complex with signal peptidase. J. Am. Chem. Soc. 133, 17869–17877 (2011).
Roberts, T. C., Schallenberger, M. A., Liu, J., Smith, P. A. & Romesberg, F. E. Initial efforts toward the optimization of arylomycins for antibiotic activity. J. Med. Chem. 54, 4954–4963 (2011).
Therien, A. G. et al. Broadening the spectrum of β-lactam antibiotics through inhibition of signal peptidase type I. Antimicrob. Agents Chemother. 56, 4662–4670 (2012).
Peters, D. S., Romesberg, F. E. & Baran, P. S. Scalable access to arylomycins via C–H functionalization logic. J. Am. Chem. Soc. 140, 2072–2075 (2018).
Castanheira, M., Huband, M. D., Mendes, R. E. & Flamm, R. K. Meropenem-vaborbactam tested against contemporary gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.00567–17 (2017).
Endimiani, A. et al. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 53, 4504–4507 (2009).
Lüke, I., Handford, J. I., Palmer, T. & Sargent, F. Proteolytic processing of Escherichia coli twin-arginine signal peptides by LepB. Arch. Microbiol. 191, 919–925 (2009).
Silver, L. L. Multi-targeting by monotherapeutic antibacterials. Nat. Rev. Drug Discov. 6, 41–55 (2007).
Singh, J., Petter, R. C., Baillie, T. A. & Whitty, A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 10, 307–317 (2011).
Sampson, B. A., Misra, R. & Benson, S. A. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 122, 491–501 (1989).
Silver, L. L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 24, 71–109 (2011).
Hancock, R. E. & Bell, A. Antibiotic uptake into Gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 7, 713–720 (1988).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Richter, M. F. & Hergenrother, P. J. The challenge of converting Gram-positive-only compounds into broad-spectrum antibiotics. Ann. NY Acad. Sci. 24, 71 (2018).
Raetz, C. R. H., Reynolds, C. M., Trent, M. S. & Bishop, R. E. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76, 295–329 (2007).
Kiho, T. et al. Structure–activity relationships of globomycin analogues as antibiotics. Bioorg. Med. Chem. 12, 337–361 (2004).
Acknowledgements
We thank Confluence Discovery Technologies for running the enzymatic inactivation studies and WuXi Shanghai for determining MIC values to support the chemistry effort. We thank C. Peng, H. La, J. Z. Chen, F. Ma, X. Liang, S. Ubhayakar and the protein expression group from Genentech for supporting the research efforts required to discover G0775, J. Guillory for performing the whole-genome sequencing on CDC 0106, and B. Sellers for performing property calculations on synthesized compounds.
Reviewer information
Nature thanks E. Brown, P. Hergenrother, T. Maier and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
P.A.S., M.F.T.K., Yu.C., M.R.D., M.X., M.W.T. and C.E.H. conceived the study and managed the execution of experiments. H.S.G. and P.A.S. performed all the microbiology experiments. D.Y., J.K., S.P., M.X. and H.Z. performed all in vivo efficacy studies. J.M. and L.R. performed all crystallographic experiments. W.P. performed the proteomics experiments. C.E.H. and J.G.Q. performed enzymology experiments. M.F.T.K., P.A.S., Yo.C., J.J.C., T.C.R., R.I.H., P.P., J.B.S., J.W. and Z.Y. designed and synthesized the molecules. E.S. performed the whole-genome sequencing. P.A.S., M.F.T.K. and C.E.H. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
P.A.S., M.F.T.K., Yo.C., J.J.C., R.I.H., P.P., T.C.R., J.B.S. and Z.Y are listed as inventors on the pending patent application WO2017084630, which covers the molecules G0775, G6850, G3031 and G8126. P.A.S., M.F.T.K., Yu.C., M.R.D., M.X., M.W.T., H.S.G., D.Y., J.K., S.P., H.Z., J.M., L.R., W.P., J.G.Q., J.J.C. and C.E.H. declare active employment by Genentech at the time of submission.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Growth rates of E. coli during treatment with G0775 or when LepB expression levels are modulated.
a, Time-kill data of E. coli ATCC 25922 when treated with G0775 at 0.25, 1, 4 and 16 times the measured MIC of 0.125 µg ml−1. The data is an average of three independent experiments with error bars representing s.d. b, Western blot of V5-epitope-tagged LepB from an arabinose-inducible conditional LepB strain (E. coli UPEC CFT073) grown in the indicated sugar concentrations. This experiment was performed once. c, Growth-curve analysis of the same conditional LepB strain grown in the indicated sugar concentrations. Data is an average of triplicate data points with error bars representing s.d. The graph is a representative of three independent experiments.
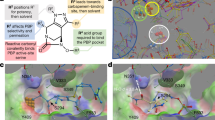
Extended Data Fig. 2 Overlay of LepB–G0775 and LepB–arylomycin A2 (PDB ID: 1T7D).
Comparison of G0775 (coloured yellow) and arylomycin (coloured pink). The catalytic lysine 146 is covalently bound to the nitrile warhead, while the serine 91 nucleophile remains unbound. For simplicity, the LepB protein has been removed from each co-structure. The comparison indicates that the macrocyclic core of G0775 maintains high similarity to the parent arylomycin macrocycle, and makes very similar interactions with the protein.
Extended Data Fig. 3 Proposed mechanism of covalent amidine-bond formation between G0775 nitrile and lysine 146.
The general base (lysine 146) involved in substrate proteolysis functions instead as a nucleophile to attack the nitrile warhead.
Extended Data Fig. 4 Details of the asymmetric unit and the electron density of the LepB–G0775 complex.
a, The LepB–G0775 complex crystallized in space group I4 with two molecules in the asymmetric unit. Chain A is coloured green, with the inhibitor shown as sticks and its carbon atoms in wheat; chain B is coloured cyan, with the inhibitor shown as sticks and its carbon atoms coloured orange. Lattice contacts across symmetry-related molecules are not shown for clarity. b, mFo−DFc omit maps for each inhibitor molecule in the asymmetric unit are shown as grey mesh contoured at 3σ.
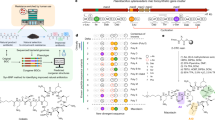
Extended Data Fig. 5 Analysis of Gram-negative outer membrane permeability for 1,069 arylomycin analogues.
a, MIC values of test compounds against E. coli ATCC 25922 and the outer-membrane-permeabilized E. coli IMP strain in the K-12 background show a consistent shift across nearly three orders of magnitude of activity. A grey line indicates a 30-fold shift in activity, and the points are coloured by the charge calculated to be the predominant species in solution at pH 7.4. Charges were calculated using MoKa 2.6.6. MIC values were measured in duplicate at a minimum, and the geometric mean was plotted. b, Box-and-whisker plot illustrating the IMP:25922 ratio of MIC values, for each indicated charge state from neutral to +4 charged compounds. c, Box-and-whisker plots showing the IMP:25922 ratio of MIC values with the compounds subdivided by molecular weight. For b and c, the central horizontal line indicates the median value for each group, and the box ends indicate the first and third quartiles in each group. The whiskers correspond to the highest non-outlier points in each group. Outliers are defined as the points outside the median value ±1.5-fold the difference between the third quartile and first quartile. Outlier points are plotted individually.
Extended Data Fig. 6
Chemical structures and biological activities of molecules along the optimization pathway from arylomycin A-C16 to G0775. MICs reported are in μg ml−1 units.
Extended Data Fig. 7 In vivo efficacy of ciprofloxacin.
Thigh infections initiated in neutropenic mice with the indicated Gram-negative bacterial species were treated with ciprofloxacin (Cipro) (n = 5 mice for E. coli and A. baumannii; n = 4 mice for K. pneumoniae and P. aeruginosa) or vehicle (n = 2 mice for P. aeruginosa, n = 4 mice for K. pneumoniae, n = 5 mice for E. coli and A. baumannii), and bacterial burden was quantified 20 h after infection. Ciprofloxacin was delivered subcutaneously once during the infection period, 2 h post-infection at the indicated dose. The pre-treatment and vehicle groups are identical to those shown in Fig. 4a, as these data were generated in the same studies. Broken red lines represent the limit of bacterial CFU determination, and the centre measure represents the mean with error bars representing s.e.m.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3, Supplementary Methods and notes on Synthetic Chemistry. Supplementary Data Table 1 shows the G0775 MIC values measured against a challenge panel of multi-drug resistant clinical isolates obtained from the Centers for Disease Control and Prevention (CDC). Supplementary Data Table 2 shows the genetic determinants of resistance identified in K. pneumoniae CDC 0106 using whole genome sequencing. Supplementary Data Table 3 shows the data collection and refinement statistics (molecular replacement) of G0775-LepB structure. Supplementary Methods shows all experimental methods used in the manuscript including: MIC, FOR, Proteomics, Protein Expression/Purification, Crystallography, Enzymology, Efficacy Studies, Time-Kill, Inducible Strain Construction, Western Blots and Cell Cytotoxicity. Synthetic Chemistry shows the synthetic schemes and analytical characterization for all molecules included in the manuscript
Rights and permissions
About this article
Cite this article
Smith, P.A., Koehler, M.F.T., Girgis, H.S. et al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature 561, 189–194 (2018). https://doi.org/10.1038/s41586-018-0483-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0483-6