Sizaire V, Nackers F, Comte E, Portaels F. Mycobacterium ulcerans infection: control, diagnosis, and treatment. Lancet Infect Dis. 2006 May. 6(5):288-96. [QxMD MEDLINE Link].
Yotsu RR, Murase C, Sugawara M, Suzuki K, Nakanaga K, Ishii N, et al. Revisiting Buruli ulcer. J Dermatol. 2015 Nov. 42 (11):1033-41. [QxMD MEDLINE Link].
World Health Organization. Buruli ulcer disease (Mycobacterium ulcerans infection). World Health Organization. Available at http://www.who.int/mediacentre/factsheets/fs199/en/. Accessed: 8/13/2016.
Walsh DS, Portaels F, Meyers WM. Buruli ulcer (Mycobacterium ulcerans infection). Trans R Soc Trop Med Hyg. 2008 Oct. 102(10):969-78. [QxMD MEDLINE Link].
Wansbrough-Jones M, Phillips R. Buruli ulcer: emerging from obscurity. Lancet. 2006 Jun 3. 367(9525):1849-58. [QxMD MEDLINE Link].
George KM, Chatterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999 Feb 5. 283(5403):854-7. [QxMD MEDLINE Link].
Walsh DS, Meyers WM, Portaels F, et al. High rates of apoptosis in human Mycobacterium ulcerans culture-positive buruli ulcer skin lesions. Am J Trop Med Hyg. 2005 Aug. 73(2):410-5. [QxMD MEDLINE Link].
Pimsler M, Sponsler TA, Meyers WM. Immunosuppressive properties of the soluble toxin from Mycobacterium ulcerans. J Infect Dis. 1988 Mar. 157(3):577-80. [QxMD MEDLINE Link].
Phillips R, Sarfo FS, Guenin-Macé L, Decalf J, Wansbrough-Jones M, Albert ML, et al. Immunosuppressive signature of cutaneous Mycobacterium ulcerans infection in the peripheral blood of patients with buruli ulcer disease. J Infect Dis. 2009 Dec 1. 200(11):1675-84. [QxMD MEDLINE Link].
Boleira M, Lupi O, Lehman L, Asiedu KB, Kiszewski AE. Buruli ulcer. An Bras Dermatol. 2010 Jun. 85(3):281-298; quiz 299-301. [QxMD MEDLINE Link]. [Full Text].
Guenin-Macé L, Veyron-Churlet R, Thoulouze MI, Romet-Lemonne G, Hong H, Leadlay PF, et al. Mycolactone activation of Wiskott-Aldrich syndrome proteins underpins Buruli ulcer formation. J Clin Invest. 2013 Apr 1. 123(4):1501-12. [QxMD MEDLINE Link]. [Full Text].
Sarfo FS, Phillips R, Wansbrough-Jones M, Simmonds RE. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016 Jan. 18 (1):17-29. [QxMD MEDLINE Link].
Phillips RO, Sarfo FS, Landier J, Oldenburg R, Frimpong M, Wansbrough-Jones M, et al. Combined inflammatory and metabolic defects reflected by reduced serum protein levels in patients with Buruli ulcer disease. PLoS Negl Trop Dis. 2014 Apr. 8 (4):e2786. [QxMD MEDLINE Link].
Anand U, Sinisi M, Fox M, MacQuillan A, Quick T, Korchev Y, et al. Mycolactone-mediated neurite degeneration and functional effects in cultured human and rat DRG neurons: Mechanisms underlying hypoalgesia in Buruli ulcer. Mol Pain. 2016. 12:[QxMD MEDLINE Link].
Ogbechi J, Ruf MT, Hall BS, Bodman-Smith K, Vogel M, Wu HL, et al. Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions. PLoS Pathog. 2015 Jul. 11 (7):e1005011. [QxMD MEDLINE Link].
Stienstra Y, van der Werf TS, Oosterom E, et al. Susceptibility to Buruli ulcer is associated with the SLC11A1 (NRAMP1) D543N polymorphism. Genes Immun. 2006 Apr. 7(3):185-9. [QxMD MEDLINE Link].
Vogel M, Bayi PF, Ruf MT, Bratschi MW, Bolz M, Um Boock A, et al. Local Heat Application for the Treatment of Buruli Ulcer: Results of a Phase II Open Label Single Center Non Comparative Clinical Trial. Clin Infect Dis. 2016 Feb 1. 62 (3):342-50. [QxMD MEDLINE Link].
Eddyani M, Ofori-Adjei D, Teugels G, et al. Potential role for fish in transmission of Mycobacterium ulcerans disease (Buruli ulcer): an environmental study. Appl Environ Microbiol. 2004 Sep. 70(9):5679-81. [QxMD MEDLINE Link].
Marsollier L, Severin T, Aubry J, et al. Aquatic snails, passive hosts of Mycobacterium ulcerans. Appl Environ Microbiol. 2004 Oct. 70(10):6296-8. [QxMD MEDLINE Link].
Marsollier L, Stinear T, Aubry J, et al. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl Environ Microbiol. 2004 Feb. 70(2):1097-103. [QxMD MEDLINE Link].
Raghunathan PL, Whitney EA, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans Infection): results from a case-control study in Ghana. Clin Infect Dis. 2005 May 15. 40(10):1445-53. [QxMD MEDLINE Link].
N'krumah RT, Koné B, Tiembre I, Cissé G, Pluschke G, Tanner M, et al. Socio-Environmental Factors Associated with the Risk of Contracting Buruli Ulcer in Tiassalé, South Côte d'Ivoire: A Case-Control Study. PLoS Negl Trop Dis. 2016 Jan. 10 (1):e0004327. [QxMD MEDLINE Link].
Jacobsen KH, Padgett JJ. Risk factors for Mycobacterium ulcerans infection. Int J Infect Dis. 2010 Aug. 14(8):e677-81. [QxMD MEDLINE Link].
Meyers WM, Shelly WM, Connor DH, Meyers EK. Human Mycobacterium ulcerans infections developing at sites of trauma to skin. Am J Trop Med Hyg. 1974 Sep. 23(5):919-23. [QxMD MEDLINE Link].
Landier J, Boisier P, Fotso Piam F, Noumen-Djeunga B, Simé J, Wantong FG, et al. Adequate wound care and use of bed nets as protective factors against Buruli Ulcer: results from a case control study in Cameroon. PLoS Negl Trop Dis. 2011 Nov. 5(11):e1392. [QxMD MEDLINE Link]. [Full Text].
Johnson PD, Azuolas J, Lavender CJ, et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007 Nov. 13(11):1653-60. [QxMD MEDLINE Link].
Fyfe JA, Lavender CJ, Handasyde KA, Legione AR, O'Brien CR, Stinear TP, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2010 Aug 10. 4(8):e791. [QxMD MEDLINE Link]. [Full Text].
Merritt RW, Walker ED, Small PL, Wallace JR, Johnson PD, Benbow ME, et al. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis. 2010 Dec 14. 4(12):e911. [QxMD MEDLINE Link]. [Full Text].
Wilson MD, Boakye DA, Mosi L, Asiedu K. In the case of transmission of mycobacterium ulcerans in buruli ulcer disease acanthamoeba species stand accused. Ghana Med J. 2011 Mar. 45(1):31-4. [QxMD MEDLINE Link].
Ohtsuka M, Kikuchi N, Yamamoto T, Suzutani T, Nakanaga K, Suzuki K, et al. Buruli ulcer caused by Mycobacterium ulcerans subsp shinshuense: a rare case of familial concurrent occurrence and detection of insertion sequence 2404 in Japan. JAMA Dermatol. 2014 Jan. 150 (1):64-7. [QxMD MEDLINE Link].
Silva MT, Portaels F, Pedrosa J. Pathogenetic mechanisms of the intracellular parasite Mycobacterium ulcerans leading to Buruli ulcer. Lancet Infect Dis. 2009 Nov. 9(11):699-710. [QxMD MEDLINE Link].
Timothy JWS, Pullan RL, Yotsu RR. Methods and Approaches for Buruli Ulcer Surveillance in Africa: Lessons Learnt and Future Directions. Methods Mol Biol. 2022. 2387:87-102. [QxMD MEDLINE Link].
O'Brien DP, Friedman ND, Cowan R, Pollard J, McDonald A, Callan P, et al. Mycobacterium ulcerans in the Elderly: More Severe Disease and Suboptimal Outcomes. PLoS Negl Trop Dis. 2015 Dec. 9 (12):e0004253. [QxMD MEDLINE Link].
Chany AC, Tresse C, Casarotto V, Blanchard N. History, biology and chemistry of Mycobacterium ulcerans infections (Buruli ulcer disease). Nat Prod Rep. 2013 Dec. 30(12):1527-67. [QxMD MEDLINE Link].
Klis S, Ranchor A, Phillips RO, Abass KM, Tuah W, Loth S, et al. Good quality of life in former Buruli ulcer patients with small lesions: long-term follow-up of the BURULICO trial. PLoS Negl Trop Dis. 2014 Jul. 8 (7):e2964. [QxMD MEDLINE Link].
Trubiano JA, Lavender CJ, Fyfe JA, Bittmann S, Johnson PD. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS Negl Trop Dis. 2013. 7(10):e2463. [QxMD MEDLINE Link]. [Full Text].
Landier J, Constantin de Magny G, Garchitorena A, Guégan JF, Gaudart J, Marsollier L, et al. Seasonal Patterns of Buruli Ulcer Incidence, Central Africa, 2002-2012. Emerg Infect Dis. 2015 Aug. 21 (8):1414-7. [QxMD MEDLINE Link].
[Guideline] Guidance on sampling techniques for laboratory-confirmation of Mycobacterium ulcerans infection (Buruli ulcer disease). Available at http://www.who.int/buruli/Guidance_sampling_techniques_MU_infection.pdf?ua=1.
O'Brien DP, Walton A, Hughes AJ, Friedman ND, McDonald A, Callan P, et al. Risk factors for recurrent Mycobacterium ulcerans disease after exclusive surgical treatment in an Australian cohort. Med J Aust. 2013 May 6. 198(8):436-9. [QxMD MEDLINE Link].
Mueller YK, Bastard M, Nkemenang P, Comte E, Ehounou G, Eyangoh S, et al. The "Buruli Score": Development of a Multivariable Prediction Model for Diagnosis of Mycobacterium ulcerans Infection in Individuals with Ulcerative Skin Lesions, Akonolinga, Cameroon. PLoS Negl Trop Dis. 2016 Apr. 10 (4):e0004593. [QxMD MEDLINE Link].
Pommelet V, Vincent QB, Ardant MF, Adeye A, Tanase A, Tondeur L, et al. Findings in patients from Benin with osteomyelitis and polymerase chain reaction-confirmed Mycobacterium ulcerans infection. Clin Infect Dis. 2014 Nov 1. 59 (9):1256-64. [QxMD MEDLINE Link].
Ruf MT, Bolz M, Vogel M, Bayi PF, Bratschi MW, Sopho GE, et al. Spatial Distribution of Mycobacterium ulcerans in Buruli Ulcer Lesions: Implications for Laboratory Diagnosis. PLoS Negl Trop Dis. 2016 Jun. 10 (6):e0004767. [QxMD MEDLINE Link].
Walsh DS, Portaels F, Meyers WM. Buruli ulcer: Advances in understanding Mycobacterium ulcerans infection. Dermatol Clin. 2011 Jan. 29(1):1-8. [QxMD MEDLINE Link].
Herbinger KH, Adjei O, Awua-Boateng NY, Nienhuis WA, Kunaa L, Siegmund V, et al. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin Infect Dis. 2009 Apr 15. 48(8):1055-64. [QxMD MEDLINE Link].
Herbinger KH, Beissner M, Huber K, Awua-Boateng NY, Nitschke J, Thompson W, et al. Efficiency of fine-needle aspiration compared with other sampling techniques for laboratory diagnosis of Buruli ulcer disease. J Clin Microbiol. 2010 Oct. 48(10):3732-4. [QxMD MEDLINE Link]. [Full Text].
Phillips R, Horsfield C, Kuijper S, et al. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an Assay using punch biopsy specimens for diagnosis of Buruli ulcer. J Clin Microbiol. 2005 Aug. 43(8):3650-6. [QxMD MEDLINE Link].
Rondini S, Mensah-Quainoo E, Junghanss T, Pluschke G. What does detection of Mycobacterium ulcerans DNA in the margin of an excised Buruli ulcer lesion tell us?. J Clin Microbiol. 2006 Nov. 44(11):4273-5. [QxMD MEDLINE Link].
Yeboah-Manu D, Asante-Poku A, Asan-Ampah K, Ampadu ED, Pluschke G. Combining PCR with microscopy to reduce costs of laboratory diagnosis of Buruli ulcer. Am J Trop Med Hyg. 2011 Nov. 85(5):900-4. [QxMD MEDLINE Link]. [Full Text].
de Souza DK, Quaye C, Mosi L, Addo P, Boakye DA. A quick and cost effective method for the diagnosis of Mycobacterium ulcerans infection. BMC Infect Dis. Jan 2012. 12:Jan 18. [QxMD MEDLINE Link]. [Full Text].
Siegmund V, Adjei O, Nitschke J, et al. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin Infect Dis. 2007 Jul 1. 45(1):68-75. [QxMD MEDLINE Link].
Gordon CL, Buntine JA, Hayman JA, Lavender CJ, Fyfe JA, Hosking P, et al. All-oral antibiotic treatment for buruli ulcer: a report of four patients. PLoS Negl Trop Dis. 2010 Nov 30. 4(11):e770. [QxMD MEDLINE Link]. [Full Text].
Eddyani M, Vandelannoote K, Meehan CJ, Bhuju S, Porter JL, Aguiar J, et al. A Genomic Approach to Resolving Relapse versus Reinfection among Four Cases of Buruli Ulcer. PLoS Negl Trop Dis. 2015 Nov. 9 (11):e0004158. [QxMD MEDLINE Link].
Leigheb G, Cammarota T, Zavattaro E, et al. Ultrasonography for the monitoring of subcutaneous damage in Mycobacterium ulcerans infection (Buruli ulcer). Ultrasound Med Biol. 2008 Oct. 34(10):1554-63. [QxMD MEDLINE Link].
Guarner J, Bartlett J, Whitney EA, Raghunathan PL, Stienstra Y, Asamoa K, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003 Jun. 9 (6):651-656. [QxMD MEDLINE Link].
Converse PJ, Nuermberger EL, Almeida DV, Grosset JH. Treating Mycobacterium ulcerans disease (Buruli ulcer): from surgery to antibiotics, is the pill mightier than the knife?. Future Microbiol. 2011 Oct. 6(10):1185-98. [QxMD MEDLINE Link]. [Full Text].
Converse PJ, Almeida DV, Nuermberger EL. Drug Efficacy Testing in the Mouse Footpad Model of Buruli Ulcer. Methods Mol Biol. 2022. 2387:195-207. [QxMD MEDLINE Link].
[Guideline] World Health Organization. Treatment of mycobaterium ulcerans disease (Buruli ulcer): guidance for health workers:. World Health Organization. Available at http://ttp://www.who.int/iris/bitstream/10665/77771/1/9789241503402_eng.pdf. Accessed: March 2013.
Chauty A, Ardant MF, Adeye A, et al. Promising clinical efficacy of streptomycin-rifampin combination for treatment of buruli ulcer (Mycobacterium ulcerans disease). Antimicrob Agents Chemother. 2007 Nov. 51(11):4029-35. [QxMD MEDLINE Link].
Etuaful S, Carbonnelle B, Grosset J, et al. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother. 2005 Aug. 49(8):3182-6. [QxMD MEDLINE Link].
Sarfo FS, Phillips R, Asiedu K, Ampadu E, Bobi N, Adentwe E, et al. Clinical efficacy of combination of rifampin and streptomycin for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother. 2010 Sep. 54(9):3678-85. [QxMD MEDLINE Link]. [Full Text].
Streptomycin: Drug information Lexicomp. UpToDate. Available at https://www.uptodate.com/contents/streptomycin-drug-information?source=search_result&search=streptomycin&selectedTitle=1~101. 2016; Accessed: 08/14/2016.
Nienhuis WA, Stienstra Y, Thompson WA, et al. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet. 2010 Feb 20. 375(9715):664-72. [QxMD MEDLINE Link].
Johnson PD, Hayman JA, Quek TY, et al. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med J Aust. 2007 Jan 15. 186(2):64-8. [QxMD MEDLINE Link].
Friedman ND, Athan E, Hughes AJ, Khajehnoori M, McDonald A, Callan P, et al. Mycobacterium ulcerans disease: experience with primary oral medical therapy in an Australian cohort. PLoS Negl Trop Dis. 2013. 7(7):e2315. [QxMD MEDLINE Link]. [Full Text].
Converse PJ, Tyagi S, Xing Y, Li SY, Kishi Y, Adamson J, et al. Efficacy of Rifampin Plus Clofazimine in a Murine Model of Mycobacterium ulcerans Disease. PLoS Negl Trop Dis. 2015. 9 (6):e0003823. [QxMD MEDLINE Link].
Velding K, Klis SA, Abass KM, van der Werf TS, Stienstra Y. The Application of Modern Dressings to Buruli Ulcers: Results from a Pilot Implementation Project in Ghana. Am J Trop Med Hyg. 2016 Jul 6. 95 (1):60-2. [QxMD MEDLINE Link].
Meyers WM, Shelly WM, Connor DH. Heat treatment of Mycobacterium ulcerans infections without surgical excision. Am J Trop Med Hyg. 1974 Sep. 23(5):924-9. [QxMD MEDLINE Link].
Junghanss T, Um Boock A, Vogel M, Schuette D, Weinlaeder H, Pluschke G. Phase change material for thermotherapy of Buruli ulcer: a prospective observational single centre proof-of-principle trial. PLoS Negl Trop Dis. 2009. 3(2):e380. [QxMD MEDLINE Link]. [Full Text].
Krieg RE, Wolcott JH, Confer A. Treatment of Mycobacterium ulcerans infection by hyperbaric oxygenation. Aviat Space Environ Med. 1975 Oct. 46(10):1241-5. [QxMD MEDLINE Link].
Bertolotti A, Izzo A, Grigolato PG, Iabichella ML. The use of ozone therapy in Buruli ulcer had an excellent outcome. BMJ Case Rep. 2013 Jan 31. 2013:[QxMD MEDLINE Link].
Murase C, Kono M, Nakanaga K, Ishii N, Akiyama M. Buruli Ulcer Successfully Treated With Negative-Pressure Wound Therapy. JAMA Dermatol. 2015 Oct. 151 (10):1137-9. [QxMD MEDLINE Link].
Warryn L, Pluschke G. Efficacy of an acid-oxidising solution (AOS) against Mycobacterium ulcerans. Antimicrob Agents Chemother. 2021 Oct 18. AAC0087021. [QxMD MEDLINE Link].
Adu E, Ampadu E, Acheampong D. Surgical management of buruli ulcer disease: a four-year experience from four endemic districts in ghana. Ghana Med J. 2011 Mar. 45(1):4-9. [QxMD MEDLINE Link]. [Full Text].
Bretzel G, Siegmund V, Racz P, et al. Post-surgical assessment of excised tissue from patients with Buruli ulcer disease: progression of infection in macroscopically healthy tissue. Trop Med Int Health. 2005 Nov. 10(11):1199-206. [QxMD MEDLINE Link].
Minutilli E, Orefici G, Pardini M, et al. Squamous cell carcinoma secondary to buruli ulcer. Dermatol Surg. 2007 Jul. 33(7):872-5. [QxMD MEDLINE Link].
Beissner M, Arens N, Wiedemann F, Piten E, Kobara B, Bauer M, et al. Treatment Outcome of Patients with Buruli Ulcer Disease in Togo. PLoS Negl Trop Dis. 2015. 9 (10):e0004170. [QxMD MEDLINE Link].
Pommelet V, Vincent QB, Ardant MF, Adeye A, Tanase A, Tondeur L, et al. Findings in patients from Benin with osteomyelitis and polymerase chain reaction-confirmed Mycobacterium ulcerans infection. Clin Infect Dis. 2014 Nov 1. 59 (9):1256-64. [QxMD MEDLINE Link].
O'Brien DP, Robson ME, Callan PP, McDonald AH. "Paradoxical" immune-mediated reactions to Mycobacterium ulcerans during antibiotic treatment: a result of treatment success, not failure. Med J Aust. 2009 Nov 16. 191(10):564-6. [QxMD MEDLINE Link].
O'Brien DP, Robson M, Friedman ND, Walton A, McDonald A, Callan P, et al. Incidence, clinical spectrum, diagnostic features, treatment and predictors of paradoxical reactions during antibiotic treatment of Mycobacterium ulcerans infections. BMC Infect Dis. 2013 Sep 5. 13:416. [QxMD MEDLINE Link]. [Full Text].
Friedman ND, McDonald AH, Robson ME, O'Brien DP. Corticosteroid use for paradoxical reactions during antibiotic treatment for Mycobacterium ulcerans. PLoS Negl Trop Dis. 2012. 6(9):e1767. [QxMD MEDLINE Link]. [Full Text].
Barogui YT, Klis SA, Johnson RC, Phillips RO, van der Veer E, van Diemen C, et al. Genetic Susceptibility and Predictors of Paradoxical Reactions in Buruli Ulcer. PLoS Negl Trop Dis. 2016 Apr. 10 (4):e0004594. [QxMD MEDLINE Link].
Phillips RO, Frimpong M, Sarfo FS, Kretschmer B, Beissner M, Debrah A, et al. Infection with Mansonella perstans Nematodes in Buruli Ulcer Patients, Ghana. Emerg Infect Dis. 2014 Jun. 20 (6):1000-3. [QxMD MEDLINE Link].
Wanda F, Nkemenang P, Ehounou G, Tchaton M, Comte E, Toutous Trellu L, et al. Clinical features and management of a severe paradoxical reaction associated with combined treatment of Buruli ulcer and HIV co-infection. BMC Infect Dis. 2014 Jul 30. 14:423. [QxMD MEDLINE Link].
Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. Long term streptomycin toxicity in the treatment of Buruli Ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis. 2014 Mar. 8 (3):e2739. [QxMD MEDLINE Link].
Nackers F, Dramaix M, Johnson RC, et al. BCG vaccine effectiveness against Buruli ulcer: a case-control study in Benin. Am J Trop Med Hyg. 2006 Oct. 75(4):768-74. [QxMD MEDLINE Link].
van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005 Oct. 83(10):785-91. [QxMD MEDLINE Link].
Abass KM, van der Werf TS, Phillips RO, Sarfo FS, Abotsi J, Mireku SO, et al. Buruli ulcer control in a highly endemic district in Ghana: role of community-based surveillance volunteers. Am J Trop Med Hyg. 2015 Jan. 92 (1):115-7. [QxMD MEDLINE Link].
Phanzu DM, Suykerbuyk P, Imposo DB, Lukanu PN, Minuku JB, Lehman LF, et al. Effect of a control project on clinical profiles and outcomes in buruli ulcer: a before/after study in Bas-Congo, Democratic Republic of Congo. PLoS Negl Trop Dis. 2011 Dec. 5(12):e1402. [QxMD MEDLINE Link]. [Full Text].
Buruli ulcer: first programme review meeting for west Africa--summary report. Wkly Epidemiol Rec. 2009 Feb 6. 84(6):43-8. [QxMD MEDLINE Link].
Ackumey MM, Kwakye-Maclean C, Ampadu EO, de Savigny D, Weiss MG. Health services for Buruli ulcer control: lessons from a field study in Ghana. PLoS Negl Trop Dis. 2011 Jun. 5(6):e1187. [QxMD MEDLINE Link]. [Full Text].
[Guideline] Valérie Simonet. Prevention of disability in Buruli ulcer:basic rehabilitationPractical field guide. Available at http://whqlibdoc.who.int/hq/2008/WHO_HTM_NTD_IDM_GBUI_2008.1_eng.pdf. Accessed: May 28 2012.
Barclay L. IDSA: skin and soft tissue infections guidelines updated. Medscape Medical News. Available at http://www.medscape.com/viewarticle/827399. Accessed: June 26, 2014.
[Guideline] Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of america. Clin Infect Dis. 2014 Jul 15. 59(2):e10-52. [QxMD MEDLINE Link]. [Full Text].
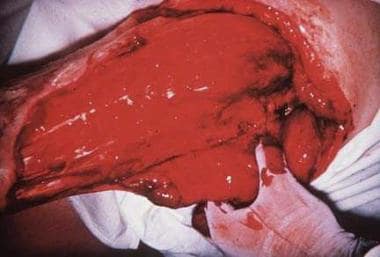 Buruli ulcer can extend to 15% of a person's skin surface and may destroy nerves and blood vessels. Metastatic bone lesions may develop.
Buruli ulcer can extend to 15% of a person's skin surface and may destroy nerves and blood vessels. Metastatic bone lesions may develop.






