The Use of Process Analytical Technology in Biofuels Production
Biofuels such as biodiesel and bioethanol are now the main alternatives to fossil fuels in one of the most pollutant human activities: transportation. The authors report on the use of process analytical technology for mapping raw materials, fingerprinting process trajectories, and calibrating for the most important quality specifications, both for individual chemical and physical attributes or for combined quality attributes, thusleading to more consistent and economically viable processes.
Process analytical technology (PAT) (1) is a landmark in the acceptance of process systems engineering tools in modern biopharmaceutical and pharmaceutical manufacturing. It is often said that PAT has already matured in many other processing areas and that it is up to industries like pharma to adopt those best-established practices. In actuality, this is not completely true. Many chemical processes use on-line monitoring for control purposes, but the in-process control specifications (IPC) measured are typically univariate and limited in information content about the product and process (for example, temperature or single compositions). By contrast, the aim of PAT is to use high-level quality specifications typically obtained by multiparametric in-situ, on-line techniques such as process spectroscopies (for example, manipulating a process based upon the entire sample matrix spectra and not a single compound signal).
Furthermore, any PAT strategy developed for a dynamic process whose trajectory strongly depends upon the starting conditions (for example, complex and natural raw materials, as in biofuel production) must take into account information from previous processing stages if a precise endpoint is to be achieved consistently after processing.
One possible and obvious general strategy to develop PAT applications should therefore involve monitoring intensification (that is, multiple quality specifications measured simultaneously and more frequently in all relevant processing steps); tools to pull together the information of several processing batches (building a design space); and systems engineering tools to analyze several runs of the entire process (thus describing the interactions between process components). We have developed and applied such a strategy successfully in the biomanufacturing of small and large molecules for some years already (2,3) and have tested its general value in other processes also involving natural and complex starting materials (crude oil refining, biodiesel production, beer brewing, and so forth) (4–7).
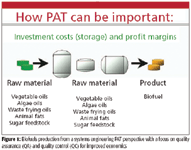
Figure 1
Biofuels are produced in a sequence of large batch operations involving multiple phases, a biochemical reaction, and several separation–purification steps. The final product must comply with multiple quality specifications despite the variability in the raw materials and the complexity in the unit operations used in their processing. Overall, biofuels production is amenable to PAT implementation in the same manner as a pharmaceutical process — given the correlation between stages and the carryover of fingerprints — or even more so if one examines process economics and its associated logistics. In fact, a recent study by the U.S. Department of Agriculture (8) has shown that storage tanks make up for one third of new biodiesel facility construction costs. So streamlining raw material acceptance and final product release (shipping out) would mean that storage capacity and its associated costs could be cut down from about a month to a few days. Also, the final product cost structure shows that there is a strong case in favor of using PAT throughout raw materials qualification, production process monitoring and supervision, and end-product multiparametric release (Figure 1). The logistics associated with the production of large quantities of a commodity-type product by batch operations, with strict quality specifications, increases the need to reduce end-product variability as well to use fast multiparametric quality control to achieve safe and fast release and reduce inventory. Once an overall quantitative description of a process is in place, a PAT strategy for closed loop control and overall process management can be devised based upon the elements in Figure 2, applied on a plantwide perspective.
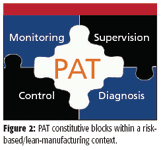
Figure 2
Process Monitoring
Large volumes of highly informative data are of course much easier to obtain with on-line multiparametric techniques (for example, in-situ spectroscopy) than with fast at-line monoparametric techniques. Multiparametric techniques, which more than monitoring a limited number of parameters are able to take a whole sample's matrix fingerprint, have the potential to capture the combined effects of important chemical and physical attributes of a sample. One such technique is near-infrared spectroscopy (NIR), which is a widely known method for its capacity for accurate process monitoring of complex matrices (9). NIR is a highly versatile technique with a well-established process monitoring track record in several processing industries. Provided that a person proficient in chemometrics is involved, who also knows about the process (that is, its dynamics and main influencing factors and sources of variability), robust and accurate calibrations can be established that will be valid across different process scales or similar-in-nature processing steps (3–7,10) (Table I). The road less traveled in this area though is the use of NIR as a process fingerprinting technique, taking advantage of its intrinsic ability to capture a sample's chemical and physical information, thus avoiding the need for calibration development and making the most of the availability of on-line in-situ measurements for batch supervision (Figure 3).

Table I
Process Supervision
Process supervision takes into consideration several types of variables at once (that is, it is multivariate) as opposed to monitoring. Process supervision is not only monitoring in perspective (the incoming monitored data point for a variable is plotted and compared with that variable's history along the batch); it is a high-level way of comparing the running batch against previous batches (for example, mapping the running batch trajectory over the nominal batch trajectory). While taking a batch perspective by analyzing several historical batches in a consolidated manner, PAT as a process supervision tool has significant potential in process analysis and understanding. If in addition to historical batches with built-in nominal variability (acceptable variability) non-nominal batches are available (that is, out of specification, abnormal, or designed batches), a broader operating region can be explored. In this case, a very reliable picture can be drawn of the process by establishing the interrelation among very diverse process and product variables, highlighting different phenomena and process kinetics (process phases). Essential concepts in the PAT context are those of quality by process design, and design, operating, and control spaces (1,11). The application of such concepts offers different opportunities depending upon the regulatory oversight of specific industries. It seems, however, that the pharmaceutical industry is misguided by imposing constraints in the operating space (space spanned by operating and manipulated variables) as opposed to achieving the highest performance possible in the control space (imposing constraints around the desired multivariate target made by multiple quality specifications). The biofuel industry, on the contrary, has not yet realized the enormous potential in leaning its manufacturing operations toward PAT and the correct control paradigm well established in the chemical processing industries for at least 20 years: that operating variables are to be manipulated based upon monitoring the multivariate quality specifications control space (11). We have demonstrated at industrial-pilot conditions the capabilities of in-process NIR for both process monitoring and supervision (10) and in similar to real conditions in large enough design spaces (4–7). Figure 4 shows that from start to end, the transesterification step can be monitored by in-situ on-line NIR.
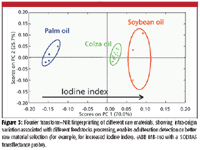
Figure 3
PAT: A Systems Perspective
As an overall picture emerges of the entire multistage process, based upon a more accurate picture of its constitutive unit operations, integration of this distributed knowledge into a plant- or process-wide perspective is of significant value in the PAT context. In fact, this can enable the anticipation and solving of problems that might have a negative impact downstream (for example, accepting or rejecting a certain raw-material lot or simply deciding on a blend of different lots) (3). Finally, the use of PAT is not limited to existing processes and products but is especially attractive in the scale-up and research and development of new processes and products. PAT is especially effective in scale-up. Because PAT involves consideration of all monitored variables and not only an empirical selection of some of those variables, and since in-process monitoring techniques are normally multiparametric (for example, NIR spectra of a whole sample), they will be more suited to capturing scale effects present in the sample's matrix that show up clearly in a consolidated multivariate analysis of quality and operating variables. This, in turn, will help the skillful engineer or scientist to pinpoint and solve scale-up problems, resulting in much faster and safer process development efforts.
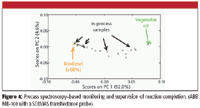
Figure 4
Conclusion
The potential of PAT in manufacturing areas other than the pharmaceutical industry is far from properly exploited, mainly due to the insufficient use of intrinsically multiparametric monitoring tools (i.e., NIR), the disregarding or ineffective use of available process information (data on historical batches or on raw-material lots), and the lack of a process- or plant-wide perspective for the proposed PAT strategy. Figure 5 points out possible answers to Figure 1, thus summarizing what can be achieved with PAT in biofuel production.

Figure 5
Acknowledgments
PF thanks The Portuguese National Science Foundation (FCT) for his Ph.D. grant (SFRH/BDE/15566/2005). We thank three of the major biodiesel suppliers in Portugal. Iberol SA and Space SA for supplying industrial samples of biodiesel and raw-materials oils; Biovegetal SA for allowing us to test in their industrial pilot-plant some of our proposed concepts. We dedicate this paper to our past and present M.Sc. and Ph.D. students whose names appear in our references.
References
(1) FDA, Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance (2004). Accessed April 7, 2008 (www.fda.gov/CDER/guid-ance/6419fnl.pdf).
(2) J.A. Lopes, J.C. Menezes, J.A. Westerhuis, and A.K. Smilde, Biotechnol. Bioeng. 80, 419–427 (2002).
(3) J.C. Menezes, A.P. Ferreira, L.O. Rodrigues, L.P. Bras, and T.P. Alves, Chemometrics Role within the PAT Context: Examples from Primary Pharmaceutical Manufacturing, chapter in Comprehensive Chemometrics (Elsevier, UK, 2008), in press.
(4) P. Baptista, P. Felizardo, J.C. Menezes, and M.J.N. Correia, Anal. Chim. Acta 607, 153–159 (2008).
(5) P. Felizardo, P. Baptista, M.S. Uva, J.C. Menezes, and M.J. Neiva-Correia, J. NIR Spec. 15(2), 97–105 (2007).
(6) P. Baptista, P. Felizardo, J.C. Menezes, and M.J. Neiva-Correia, Monitoring the quality of oils for biodiesel production using multivariate near infrared spectroscopy models (submitted for publication in J. NIR Spec. 2008).
(7) P. Baptista, P. Felizardo, J.C. Menezes, and M.J. Neiva-Correia, Multivariate near infrared spectroscopy models for predicting the iodine value, CFPP, kinematic viscosity at 40 °C and density at 15 °C of biodiesel, Talanta (2008) (in press, doi:10.1016/j.talanta. 2008.06.001). Available online: www.sciencedirect.com/science/journal/00399140.
(8) M.J. Haas, A.J. McAloon, W.C. Yee, and T.A. Foglia, Bioresource Technol. 97, 671–678 (2006).
(9) H.W. Siesler, Ed., Near-Infrared Spectroscopy: Principles, Instruments, Applications (Wiley-VCH, Hoboken, New Jersey, 2001).
(10) P.N. Fiolhais, Near Infra-Red Spectroscopy Applied to Biodiesel Production, M.Sc. Thesis (Technical University of Lisbon, Portugal; 68 pp, English, supervised by J.C. Menezes and M.J.N. Correia, 2007).
(11) J.F. MacGregor and M.J. Bruwer, J. Pharm Innovation, in press (DOI 10.1007/s12247-008-9023-5).
José C. Menezes, Pedro Felizardo, and M. Joana Neiva-Correia are with the Department of Chemical and Biological Engineering, Technical University of Lisbon, Lisbon, Portugal.
Standardizing Solutions to Enabling Platforming of Process Analytical Technology (PAT) Deployments
April 24th 2023Gary McGeorge, Scientific Director at Bristol-Myers Squibb, spoke to us of the benefits and challenges associated with establishment of consistent resolutions while facilitating the steps associated with the implementation of process analytical technology (PAT).
Determination of the Degree of Cure of a Varnish
January 8th 2016Modern paints and varnishes are complex mixtures and the optimization of the material properties is an important task. One of the most essential variables is the curing which should in most cases take place in a reasonably short time span that can range from a fraction of a second to many days. It is difficult to evaluate the degree of the curing and its completeness by visual inspection. Therefore, an unbiased and reliable quantitative analysis is needed in quality control but also in R&D to optimize the varnish or the curing method respectively.
Analysis of Solar Silicon Using High-Throughput Spectroscopy
August 1st 2009Infrared spectroscopy is a powerful analysis technique used in the semiconductor industry to ensure the quality of silicon and silicon wafers. The authors discuss the use of an inexpensive, lab-based system to measure carbon and oxygen concentrations in silicon to the level of precision required by the solar silicon industry.
