-
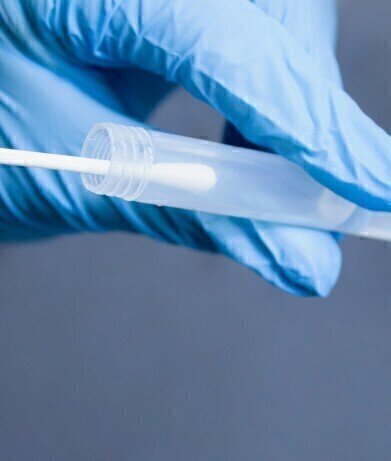 Patented PrimeStore® MTM technology enables safe sample handling and testing for multiple pathogens from one sample swab.
Patented PrimeStore® MTM technology enables safe sample handling and testing for multiple pathogens from one sample swab.
Laboratory Products
Novel Molecular Transport Media for Dual COVID-19 and Influenza Sampling Introduced
Aug 06 2020
EKF Diagnostics announces that it has added a novel viral transport media for the safe sample handling and testing of multiple infectious diseases from a single swab to its product range. PrimeStore® MTM (Molecular Transport Medium) is an FDA cleared and CE IVD marked sample collection device which deactivates viruses, including COVID-19, flu A, flu B, HIV and TB. Already proven in the US market, this fully patented device ensures transportation and workplace safety for disease testing programmes, greatly reducing risk of infection to health workers, couriers, and lab technicians.
Designed and optimised for molecular testing, PrimeStore MTM is a unique FDA class 2 cleared sample collection and transportation device that effectively kills viral and bacterial pathogens whilst preserving and stabilising the released DNA and RNA. The molecular transport media supports many downstream molecular applications, including qPCR and next generation sequencing. The sample can be safely stored at ambient temperature for up to 7 days and re-used several times. As well as reducing the risks of handling live pathogenic samples, this also cuts costs by eliminating cold chain requirement and need for Category 3 facilities, as testing can take place outside of containment.
As multiple tests can be processed from one sample swab, this makes it ideal for the approaching flu season since samples stored in PrimeStore MTM can be tested for both COVID-19 and influenza from a single sample that has been inactivated and stabilised. This can assist with disease differentiation at reduced costs.
PrimeStore MTM is proven technology with millions of tubes already sold globally during and prior to the COVID-19 pandemic to hospitals and laboratories who trust that it provides safer and more reliable testing than traditional VTM or universal transport media, saline, or RUO devices. Multiple peer-reviewed scientific papers have been published from 2011 onwards, validating the use of PrimeStore MTM for reliable pathogen detection from a wide variety of clinical matrices and biofluid types, and with a range of analytical platforms. A selection of these references are available on the EKF website.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
May 21 2024 Lagos, Nigeria
May 22 2024 Basel, Switzerland
Scientific Laboratory Show & Conference 2024
May 22 2024 Nottingham, UK
May 23 2024 Beijing, China
May 28 2024 Tel Aviv, Israel




.jpg)